Chinese scientists have reported dumbbell-shaped nanoparticles capable of detecting cyanide concentrations as low as 4 x 10-7 M in drinking water. This low detection limit, which is below the acceptable limit advised by the World Health Organization, is attributed to a combination of fluorescence detection with a filtering process called ‘magnetic concentration-washing’.
The toxicity of cyanide and its potential prevalence in drinking water has made easy detection of cyanide an active area of research. Gold nanoparticles have attracted attention as cyanide sensors due to their selectivity for cyanide over other anions. Now, Shaojun Dong and colleagues at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, have adapted a bifunctional nanoparticle made up of Au and magnetic Fe3O4 nanoparticles for sensitive detection of cyanide, even in the presence of interfering species.

The group detected cyanide by relying on what is known as the ‘inner filter effect’, observed when two molecules present in a solution have overlapping absorption and emission wavelengths. The fluorescence emission from a fluorophore is blocked, or reabsorbed, by the second molecule, reducing or even quenching the overall fluorescence of the solution. In the present system, the excitation and emission energy for the fluorophore Rhodamine B is quenched by the Au-Fe3O4 nanoparticles. However, addition of cyanide to the sample reduces the gold component of the nanoparticles by forming an Au(CN)2– complex. This change in the structure alters the nanoparticles’ absorbance spectra and the Rhodamine B emissions are no longer fully absorbed. The team calculated the amount of cyanide present in a sample by monitoring the change in fluorescence.
To extend this detection method for use with environmental samples, which may contain dyes or other contaminants that absorb or emit at a similar wavelength to Rhodamine B, Dong and colleagues implemented a ‘magnetic concentration-washing process’. In it, the Au-Fe3O4 nanoparticles are magnetically separated from the sample solution after they have reacted with any cyanide present in the sample. This magnetic separation retains the bifunctional nanoparticles and leaves any interfering species behind. Pure buffered water containing additional Rhodamine B is added and the fluorescence is monitored. The authors showed that two cycles of this process were enough to remove any interfering species and accurately detect cyanide levels.
To find out more, read the full article:
Dual-Functonal Au-Fe3O4 Dumbbell Nanoparticles for Sensitive and Selective Turn-on Fluorescent Detection of Cyanide Based on the Inner Filter Effect
Yueming Zhai, Lihua Jin, Ping Wang and Shaojun Dong, Chem. Commun., 2011, DOI: 10.1039/C1CC13149D
Posted on behalf of Patricia Pantoș, web writer for ChemComm.


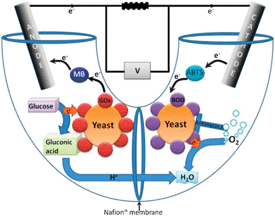









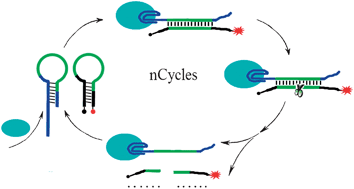 Chinese scientists have made a simple and sensitive sensor for detecting proteins, which could lead to improved disease detection.
Chinese scientists have made a simple and sensitive sensor for detecting proteins, which could lead to improved disease detection.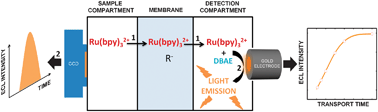

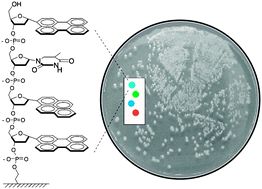
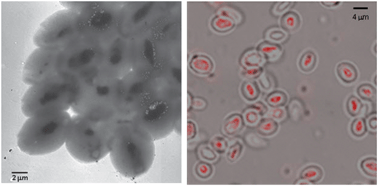
 Scientists are a step closer to understanding how an important cell organelle works, which could lead to new insight into disease such as diabetes and Alzheimer’s disease.
Scientists are a step closer to understanding how an important cell organelle works, which could lead to new insight into disease such as diabetes and Alzheimer’s disease.