What’s special about Gene delivery with polycationic fullerene hexakis-adducts?
What’s special about Gene delivery with polycationic fullerene hexakis-adducts?
Well, that’s the topic of Jean-François Nierengarten’s recent ChemComm communication, rated as ‘hot’ by the referees and free to access* until 15th March. It is also his 25th independent research article in ChemComm.
To celebrate this achievement, Professor Nierengarten has taken some time out from his research to speak to ChemComm about his career.
What inspired you to become a scientist?
As far as I remember, I was always fascinated by natural sciences and wildlife. I started to study biology at the University of Strasbourg (Université Louis Pasteur at that time) with the idea of becoming a zoologist to discover unknown animals in the Amazon rainforest or in other wild places in the world. On the way, I discovered chemistry thanks to a couple of outstanding teachers and definitively switched from biology to chemistry after I met Jean-Pierre Sauvage at the end of my first year of Master. Fortunately, after my Master, I had the chance to prepare my PhD under the guidance of Jean-Pierre, and thus to become a chemist.
What was your motivation behind the work described in your ChemComm article?
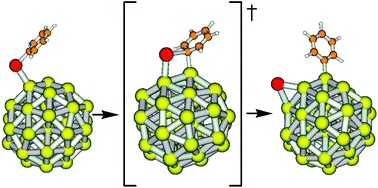
The work described in this paper is a part of our research program on the use of click chemistry for the post-functionalisation of fullerene hexa-adducts (Chem. Commun. 2008, 2450; 2010, 46, 3860 and 4160; 2011, 47, 1321). The initial driving force for this work was to apply the synthetic methodology developed in the group to the preparation of new molecules with specific properties. As very often happens, applications with our compounds rely on collaborations with colleagues having the appropriate expertise. Indeed, Jean-Serge Remy, a well-established scientist in the field of transfection and synthetic vectors, is a very good friend and discussing about science one Friday evening in a pub brought us to the idea of testing fullerene hexa-adducts as synthetic vectors. We thus prepared a series of hexa-substituted fullerene derivatives decorated with dendritic branches bearing peripheral ammonium groups. Jean-Serge and his co-workers could then show that polyplexes prepared from DNA and these globular polycationic fullerene derivatives exhibit remarkable gene delivery capabilities. This result was quite unexpected as a generally admitted rule for the design of gene delivery vectors is that compact globular polycations with an isotropic distribution of positive charges are not suitable candidates for such studies. The results reported in our ChemComm article show that this is indeed not the case.
Why did you choose ChemComm to publish your work?
For fast publication of our important findings, ChemComm is an obvious choice. Over the years, it has been always a pleasure to work with the RSC Journals in general and with ChemComm in particular. All the steps from the submission to the publication are very efficient and all is organized in a very professional way. Publishing our work in ChemComm is also the guarantee for high visibility. Finally, I am a supporter of European journals in general and strongly believe that the best of European chemistry should be reported in European journals. Having top quality journals in Europe is essential to give credit to the European chemical community.
Where do you see your research heading next?
In addition to their remarkable gene delivery capabilities, the fullerene hexa-adduct derivatives have also revealed a very low toxicity if any. The fullerene hexa-adduct core is therefore a particularly appealing 3D-scaffold for the development of new multifunctional bioactive molecules. Based on the versatile fullerene hexa-adduct building blocks already developed in our group (Chem. Commun. 2010, 46, 4160), the successive grafting of up to three different groups on the fullerene core can be efficiently achieved. We are currently working on a new generation of vectors bearing targeting subunits for specific gene delivery to selected cells and/or fluorescent probes to monitor their intracellular pathway by confocal microscopy.
What do enjoy doing in your spare time?
Spending time with Iwona, my wife, and our two kids, cooking, listening to music, travelling. I like also reading and playing the guitar but have less and less time for it!
What would you be if you weren’t a scientist?
Hopefully as happy as I am to be a scientist! I guess that it could be the case if I would be an ébéniste [cabinet maker]. During my childhood, I had a lot of fun making stuff from wood in the workshop of my godfather, a very talented ébéniste particularly gifted for marquetry. I could spend hours watching him applying pieces of veneer to form decorative patterns or pictures onto the commodes or the tables he was restoring.
Also of interest:
Less is more – multiscale modelling of self-assembling multivalency and its impact on DNA binding and gene delivery
Paola Posocco, Sabrina Pricl, Simon Jones, Anna Barnard and David K. Smith
Chem. Sci., 2010, 1, 393-404
*Access our free content any time, any place – register for an RSC Publishing personal account today