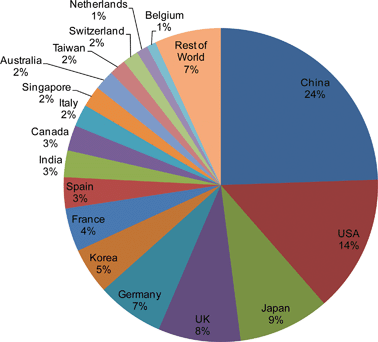
UK scientists have developed a cheap way of cleaning tarnished metals in industry to prevent corrosion using a UV activated photocatalyst ink. The dirty surface can then simply be washed away with water.
Stainless steel corrosion in industry costs the UK around 4 per cent of gross national product each year. The corrosion is caused by a build up of metal oxides on the metals’ surfaces, which can become contaminated with corrosion products. Currently, aggressive chemicals such as strong acids and chelating agents are used to remove the oxides.
Andrew Mills and David Hazafy from Queen’s University, Belfast, have made anatase titania (TiO2) films to apply to the metal surfaces to clean them. The team was initially interested in titania’s ability to drive ‘useful photo-reduction reactions’, says Mills. It was while preparing TiO2 photocatalyst films on stainless steel as part of a water-splitting photo diode project that they noticed that the appearance of tarnish was diminished.
TiO2 is a well known photocatalyst used in the redox reductions of metal oxides. When it is exposed to ultraviolet light, conduction band electrons and valence band holes are produced. The electrons and holes can either recombine or move to the surface of the film where they undergo redox reactions with adsorbed chemical species. But, on introduction of an electron donor, such as ethanol, the donor reacts with the holes, leaving the electrons free to react with metal oxides.
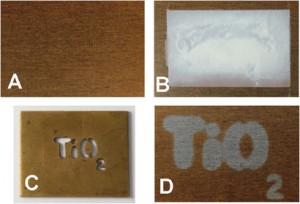
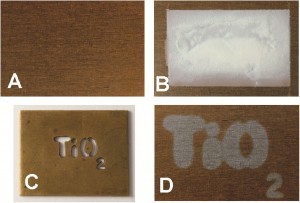
A stainless steel sample without (A) and with (B) the ink on its surface. (C): irradiation of sample B with UV light through a brass TiO2 template. (D): washing off the ink using water. The bronze coloured oxide coating has been removed by the illuminated section of the ink
Read the full story in Chemistry World
Link to journal article
UV-activated photocatalyst films and inks for cleaning tarnished metals
Andrew Mills and David Hazafy
Chem. Commun., 2012, 48, 525-527
DOI: 10.1039/C1CC15774D