A team of researchers from Shandong Normal University in Jinan, China, have developed a highly sensitive and label-free assay for the detection of uracil-DNA glycosylase, a DNA repair enzyme that removes uracil from DNA molecules. Uracil is an RNA base, and when uracil appears in DNA through deamination of cytosine or misincorporation during DNA synthesis, the error can have mutagenic consequences.
Diminished activity of uracil-DNA glycosylase has been linked to a number of disease states including human immunodeficiency and Bloom syndrome, an inherited disorder associated with an increased risk of cancer (among other symptoms). Developing sensitive methods to quantify uracil-DNA glycosylase would enable early diagnosis of such conditions and improve understanding of the DNA-repair machinery. As a proof-of-concept, the researchers showed that this method could quantify the enzyme in the cell lysate of HeLa cancer cells.
Their method reminds me of Rube Goldberg machines, which achieve a task via a series of connected, mechanical steps. Completion of one step triggers the start of another: such as a line of falling dominos hitting a marble that, in turn, rolls down a track. In this work the action of one enzyme returns a product that is the preferred substrate of another enzyme. At the risk of deviating slightly, one of the more spectacular examples of a Rube Goldberg machine is seen in the music video for OK GO’s ‘this too shall pass’, a single-take shoot of a warehouse sized machine, featuring rolling cars, swinging pianos, flowing water and rolling billiard balls, all to perform the task of (spoiler alert) blasting the band members in the face with coloured paint.
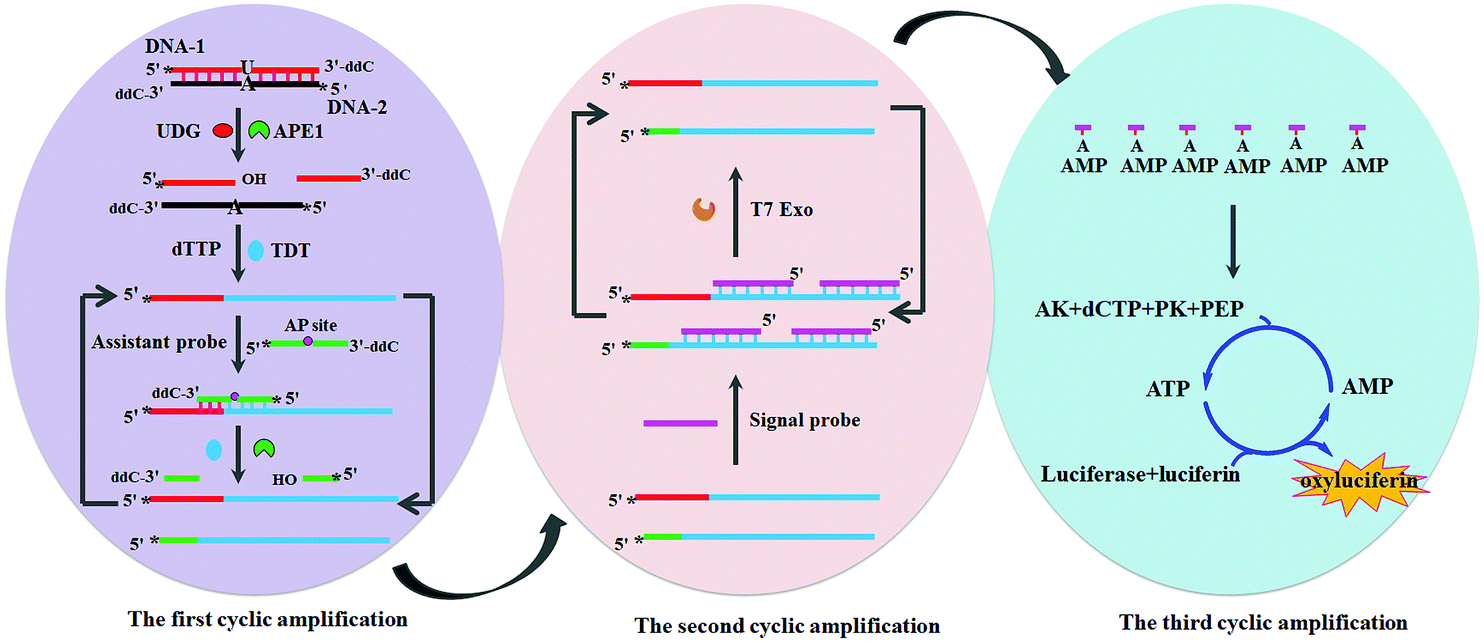
The strategy starts with the action of uracil-DNA glycosylase and ends with a bioluminescent signal via a cascade of enzymatic reactions
The authors’ strategy involves a series of sequential steps employing seven different enzymes and three nucleic acid probes. It begins with a double stranded DNA probe containing one rogue uracil base: the perfect bait for uracil-DNA glycosylase. The action of this enzyme and two others, in a process involving base excision, DNA backbone cleavage and the addition of thymine-rich sequences, produces a large quantity of single-stranded DNA molecules with long thymine-rich tails. These molecules hybridise with adenine-rich RNA probes to generate RNA-DNA duplexes. An enzyme digests the RNA portion, releasing adenosine monophosphate monomers, which are converted to adenosine triphosphate (ATP), a required energy input to activate firefly luciferase. Luciferase catalyses the oxidation of luciferin to form oxyluciferin, accompanied by a large bioluminescent signal. Thus, uracil-DNA glycosylase is detected with 1-2 orders of magnitude more sensitivity than state-of-the-art fluorescent and luminescent assays.
Unlike conventional Rube Goldberg machines, which are characterised by unnecessary complexity, in this ‘enzymatic Rube Goldberg machine’ each step has a specific purpose and serves to amplify the signal of the last. This is dubbed ‘tricyclic cascade signal amplification’ and it enables highly sensitive detection of the enzyme.
To find out more please read:
Yan Zhang, Qing-nan Li, Chen-chen Li, Chen-yang Zhang.
Chem. Commun., 2018, 54, 6991-6994
DOI: 10.1039/c8cc03769h
 About the author
About the author
Zoë Hearne is a PhD candidate in chemistry at McGill University in Montréal, Canada, under the supervision of Professor Chao-Jun Li. She hails from Canberra, Australia, where she completed her undergraduate degree. Her current research focuses on transition metal catalysis to effect novel transformations, and out of the lab she is an enthusiastic chemistry tutor and science communicator.














 Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at 


![C6CC08967D Graphical Abstract, Biological and related applications of pillar[n]arenes, Wang et al.](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6CC08967D)
 Rational control of nano-scale metal-catalysts for biomass conversion
Rational control of nano-scale metal-catalysts for biomass conversion