Entropy is not a friend of the macrocyclic chemist because creating large cyclic molecules by tying together several building blocks creates a high thermodynamic hurdle to cross. Nature has been forming these types of molecules for many millions of years and has provided inspiration for chemists to overcome these synthetic challenges. Perhaps purely for the academic interest, work began on investigating if it was possible to interlock two of these cyclic molecules together and form what is known as a catenane. Imagine a pot of spaghetti cooking and think of the strands tumbling around in the boiling water. Now imagine trying to tie all the ends together at the same time. Most will form individual rings but statistically a small number of those rings will be linked together. Conceptually this sounds easy but the reality is not so straight forward. A secondary problem is that only very small amounts of the linked rings are formed which means most of the starting material is wasted.
As interest in these curious molecules grew their potential application as switches for molecular electronics and sensors became apparent. However, if these applications were ever to be realised the cost of their formation would need to be feasible on a commercial scale. Macrocyclic chemists dream of yields over 50%, yet most chemists would be embarrassed to report a yield like this! However, we must remember that these reactions are inherently unfavourable so to achieve respectable yields we need to ‘stack the deck in our favour’ and try to make the interlocking of the rings more favourable.
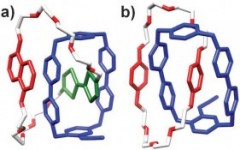
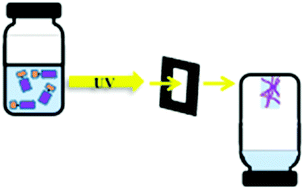
Deceptively simple but incredibly effective ways of using what are commonly thought of as weak interactions have been used to hold the two fragments together, making the subsequent formation of the interlocked rings (rather than two separate rings) much more likely. In a recent report in Chemical Communications, Fraser Stoddart and co-workers at Northwestern University, Texas A&M University and the King Abdullah City for Science and Technology report a significant development in catenane synthesis whereby yields of almost 90% are possible. As almost quantitative conversions are now being reported the commercial application of these molecules, which has for so long been discussed, moves another step closer.
Rapid thermally assisted donor–acceptor catenation
Albert C. Fahrenbach, Karel J. Hartlieb, Chi-Hau Sue, Carson J. Bruns, Gokhan Barin, Subhadeep Basu, Mark A. Olson, Youssry Y. Botros, Abdulaziz Bagabas, Nezar H. Khdary and J. Fraser Stoddart
Chem. Commun., 2012,48, 9141-9143
DOI: 10.1039/C2CC34427K