Researchers have made tremendous efforts to unlock stereoselective, catalytic organic transformations. In this recent ChemComm Feature Article, Professor Eric Meggers, one of the pioneers in the field of photoredox catalysis, provides a comprehensive review of the recent advances in asymmetric catalysis driven by visible light.
Asymmetric catalysis has been one of the most attractive yet challenging areas of organic chemistry for the synthesis of unique, biologically active natural products such as Taxol, Rapamycin, or Vinblastine that possess numerous stereocenters.
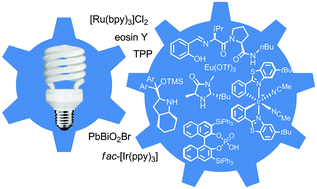
These photosensitizers are typically ruthenium or iridium complexes that can facilitate electron/energy transfer upon photoinduction. In most cases, a photoredox catalyst has to be coupled with a chiral co-catalyst to introduce stereocenters in the products.
Notable advances in the Meggers, Melchiorre, and MacMillan research groups have recently demonstrated that photoactivation can be achieved with a single chiral photosensitizer to provide products of high enantiomeric excess and good yield.
This inspirational review was just published in Chemical Communications as a Feature Article. I recommend reading “Asymmetric catalysis activated by visible light” (DOI: 10.1039/c4cc09268f) by Professor Eric Meggers to learn more about the recent advances with mechanistic details and his forecast for one of the rapidly-growing research topics in organic chemistry.
This article is free to access until 17th March.* Download it here:
Asymmetric catalysis activated by visible light
Eric Meggers �
Chem. Commun., 2015, Advance Article
DOI: 10.1039/C4CC09268F, Feature Article
Dr. Tezcan Guney is a guest web writer for Chemical Communications. Dr. Guney received his Ph.D. from the Department of Chemistry at Iowa State University with Prof. George Kraus, where he focused on the synthesis of biologically active polycyclic natural products and multifunctional imaging probes. Currently, he is a postdoctoral research scholar at the Memorial Sloan-Kettering Cancer Center in New York with Prof. Derek Tan, contributing to the efforts to access biologically active small molecules using the diversity-oriented synthetic approach.
*Access is free through a registered RSC account











 Super-dipoles uncovered in chloroform by chemists in the UK could
Super-dipoles uncovered in chloroform by chemists in the UK could  Resembling a bundle of cereal stalks, this atomic force microscopy (AFM) image depicts
Resembling a bundle of cereal stalks, this atomic force microscopy (AFM) image depicts 
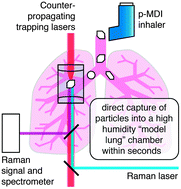
 The
The 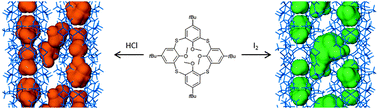
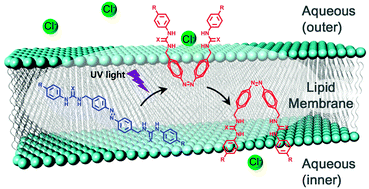
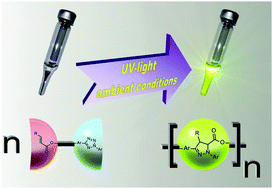


 Anthea Blackburn is a guest web writer for Chemical Communications.
Anthea Blackburn is a guest web writer for Chemical Communications.