Written by Tianyu Liu, University of California, Santa Cruz
Batteries are indispensable components that are powering a diverse array of electronics used almost every day. In recent years, due to the mass production of rechargeable electronics such as cell phones and electric vehicles, the need for reliable and economically viable batteries is rapidly increasing. Lithium-ion batteries represent a dominated rechargeable battery category that has been commercialized since early 1990s. However, the uneven distribution and high cost of lithium pose concerns on the sustainability of lithium-ion batteries.
Since the last decade, a number of scientists have shifted their attention to metal-ion batteries with more abundant and inexpensive metals than lithium, such as sodium and potassium. Change of ions calls for the need of seeking electrode materials with suitable structures that are able to host sodium or potassium ions. Most recently, Naguib and coworkers from Oak Ridge National Laboratory in USA and Purdue University in USA have identified a new two-dimensional material belonging to the MXene family that exhibits promising performance as an electrode for potassium-ion batteries. Their works has been published in Chem. Commun.
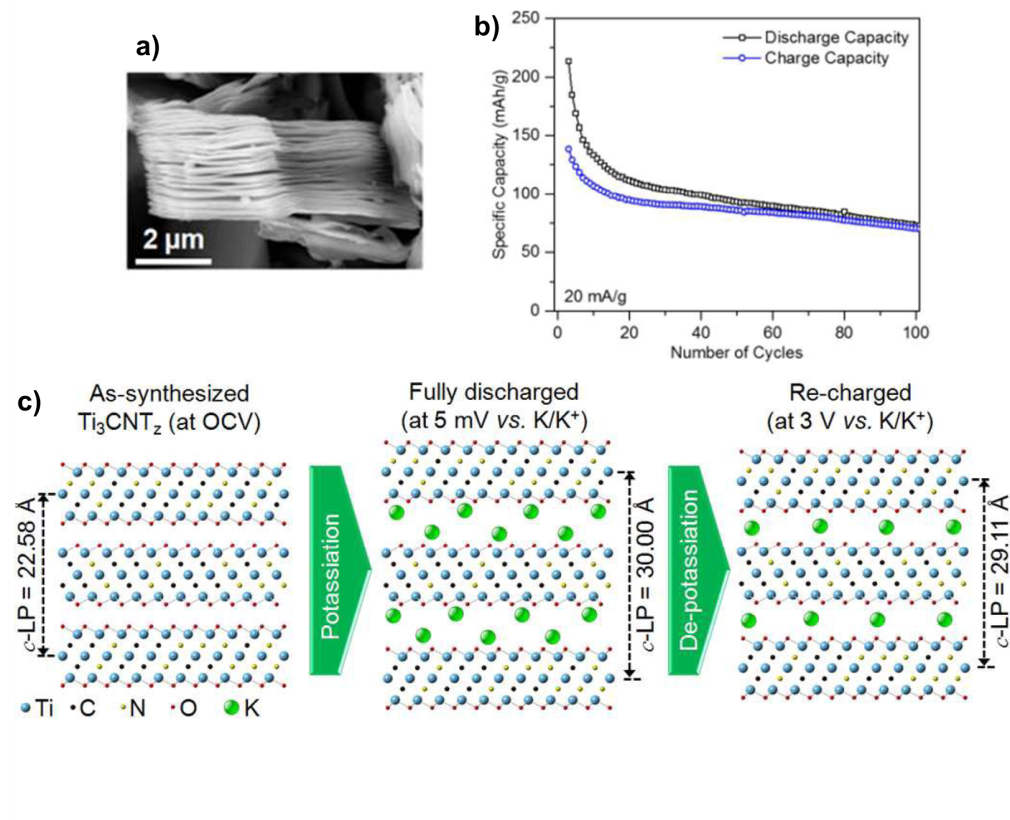
MXenes are a group of two-dimensional transition metal carbides and carbonitrides (Figure a) with chemical formula Mn+1XnTz; where M, X and Tz stand for an early transition metal element (e.g., Ti, V, Cr), carbon and/or nitrogen, and termination element (usually O, OH or F), respectively. Based on previous theoretical studies, MXenes are predicted to be capable of hosting potassium ions. In this work, Naguib et al. first synthesized one of the MXenes, Ti3CNOF, and experimentally investigated its energy storage performance.
The researchers first synthesized the Ti3CNOF powder by a wet etching process of its precursor. The obtained powder was then blended with other additives (including carbon black powder and polymer binders) and cast onto a piece of copper foil to prepare the electrode. The Ti3CNOF electrode delivered a high capacity (a measure for amount of energy that can be stored) of 710 mAh/g in the first discharging process and retained 75 mAh/g after 100 charge and discharge cycles (Figure b). In addition, the researchers gauged the charge storage mechanism of the synthesized Ti3CNOF using X-ray diffraction and X-ray photoelectron spectroscopy. The key conclusion is that potassium ions are able to intercalate in between layers of Ti3CNOF without triggering any phase change (Figure c). This mechanism is similar with lithium-ion intercalation into graphite.
Though the capacity performance reported here is not as outstanding as other graphene-based electrodes, this work provides the encouraging potential of MXenes serving as potassium-ion battery electrodes. Exploring other MXenes and modifying Ti3CNOF demonstrated here are expected to further enhance the charge storage performance of MXene-based potassium-ion batteries.
To find out more please read:
Electrochemical Performance of MXenes as K-ion Battery Anodes
Michael Naguib, Ryan A. Adams, Yunpu Zhao, Dmitry Zemlyanov, Arvind Varma, Jagjit Nanda, Vilas G. Pol.
DOI: 10.1039/C7CC02026K
About the author:
Tianyu Liu is a Ph.D. in chemistry from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.















 Scientists in the UK have developed
Scientists in the UK have developed