Written by Tianyu Liu, University of California, Santa Cruz
Chitosan is a polysaccharide derived from chitin, a second most abundant bio-polymer on earth that consists of the shells of crustaceans (such as shrimps and crabs). It is a commonly used bio-compatible material for bio-medical applications such as drug delivery.
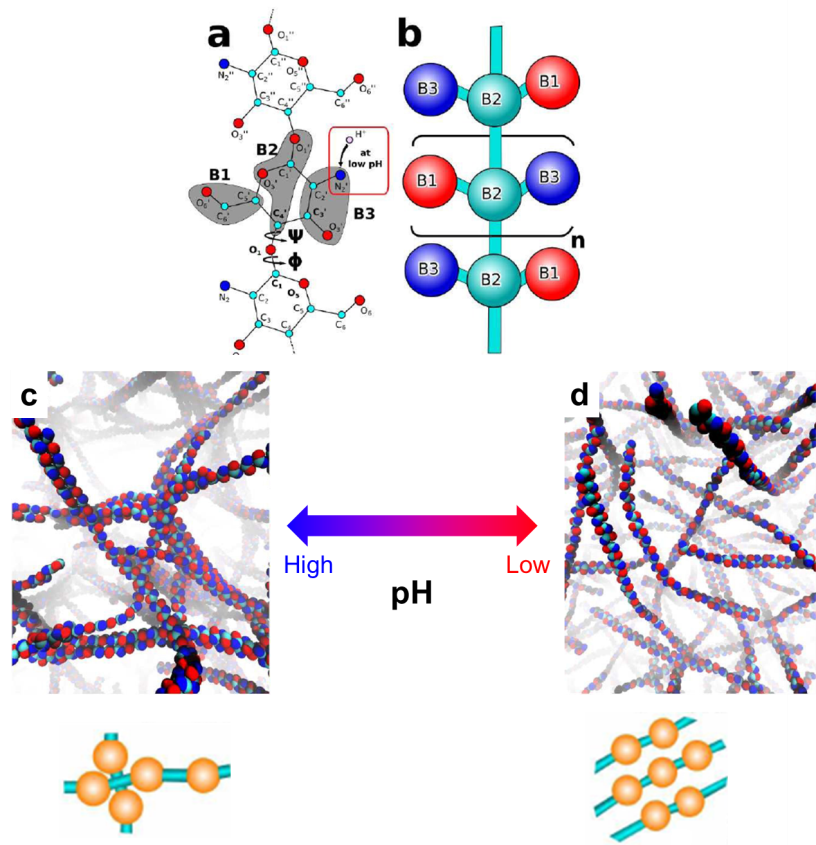
Previous studies have shown that chitosan is pH sensitive in water: it forms a viscous solution at low pH values and becomes insoluble at high pH values. Preliminary investigations suggest that such pH responsiveness is associated with the protonation and de-protonation processes of the amine groups on the chitosan polymer chain (the red box in Figure a). However, there still lacks fundamental understanding on how the pH affects the behavior of chitosan.
Now writing in Chem. Commun., Xu and Matysiak from University of Maryland, USA provided us new insights on the chitosan’s pH responsiveness at the molecular level. They adopted a method called “coarse-grained molecular simulation” to illustrate the self-assembly behaviors of chitosan polymer chains at different pH values. Unlike atomistic molecular simulations that focus on individual atoms of a molecule, the coarse-grained molecular simulation treats a group of atoms as one ensemble and probes the collective behavior of each ensemble (Figure a and b). This simulation technique demands less time than the atomistic counterparts without significantly reducing the simulation accuracy. It is suitable for characterizing polymers composed of thousands of atoms, such as chitosan.
The key discovery of this work is that the chitosan polymer chains can adopt different configurations at different pH values. At high pH values, each chain tends to crosslink perpendicularly with adjacent chains. The crosslinking reaction propagates and eventually builds up a three-dimensional dense chain network (Figure c). At low pH values, the protonated amine groups favor parallel crosslinking. Thus, each chain aligns in parallel with each other, which leads to a loosely-packed structure (Figure d). The perpendicularly cross-linked configuration reduces the solubility of chitosan in water but renders robustness and elasticity of the chitosan networks. The parallel cross-linked morphology increases water solubility but decreases the elasticity of the chitosan assembly. These conclusions obtained by the simulation are consistent with experimental results.
To find out more please read:
Effect of pH on Chitosan Hydrogel Polymer Network Structure
Hongcheng Xu and Silvina Matysiak
DOI: 10.1039/C7CC01826F
About the author:
Tianyu Liu is a Ph.D. in chemistry from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/