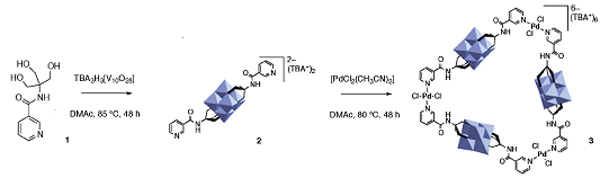
Researchers from Germany and China have designed a simple method for forming nitroarenes.
Aromatics substituted with nitro groups are ubiquitous motifs in important molecules required by the textiles, materials and pharmaceutical industries.
However, installing nitro groups can be tricky because it requires the use of strong acids or dinitrogen peroxide. These harsh conditions are not commonly tolerated by sensitive functionality present in molecules, and problems of selectivity or over-nitration commonly arise. Alternate methods use large quantities of silver salts or expensive palladium catalysts.
Now Xiao-Feng Wu (Zhejiang Sci-Tech University, China), Matthias Beller (Leibniz Institute for Catalysis, Germany) and colleagues have designed a metal-free method for nitrating aryl boronic acids.
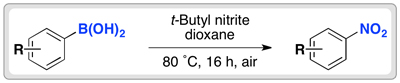
The team used an alkyl nitrite at 80 ˚C to convert the boronic acid group to a nitro group. The reaction works in moderate to good yields for electron-rich and electron-neutral aryl boronic acids. Electron-poor aromatics are more challenging substrates, as the boronic acid are less reactive.
While this method is not yet suitable for vinyl or hydroxy substituted aryl boronic acids, it offers an economically and operationally attractive method for synthesising nitro compounds.
Researcher’s perspective:Our initial investigations started with the use of a rhodium catalyst, because of rhodium’s known ability to transmetalate with arylboronic acid. Our target product, nitrobenzene, was formed but we found that the rhodium catalyst is not necessary for this transformation.
On one hand, we were disappointed, as our aim is to develop catalytic reactions; on the other hand, we are happy, because this reaction works so nicely.
Xiao-Feng Wu
Using this method, nitroarenes can be easily prepared from arylboronic acids without using catalyst and hazardous reagents.
More information can be found in the ChemComm communication, free to download for a limited period.
Posted on behalf of Alice E. Williamson, ChemComm web writer.











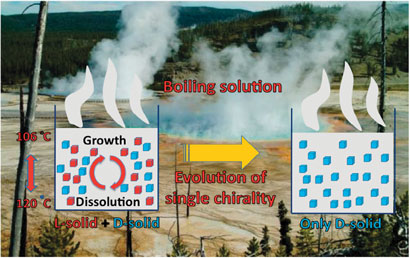 The boiling solutions in prebiotic hot springs could shed light on the emergence of a single chiral form of biomolecules in nature, say Spanish scientists.
The boiling solutions in prebiotic hot springs could shed light on the emergence of a single chiral form of biomolecules in nature, say Spanish scientists.
 55 is the age of ChemComm Associate Editor
55 is the age of ChemComm Associate Editor