Understanding metal-metal interactions is of fundamental interest to chemists, especially in the design of new materials or catalysts. Heterometallic metal-metal bonding is particularly fascinating, since the unique properties of each metal can be combined or even manipulated, to enhance structural, electronic or even photochemical effects. Gold and platinum are one such pairing of interest and there have already been several applications of AuPt clusters reported for catalysis. However, well-defined, homogeneous AuPt complexes are comparatively under explored, but there is a huge potential for these heterodinuclear complexes to catalyse useful chemical transformations. Research in Germany by Butschke and co-workers now describes the first example of an AuPt complex with a bound olefin, as a valuable, formal Au+IPt0 precursor for further reactivity and chemical transformations (Figure 1).
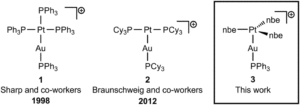
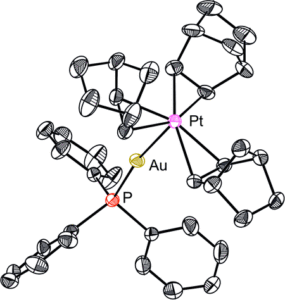
Figure 1: The new AuPt heterodinuclear complex with Pt-bound olefins (right), and existing examples in the literature with Pt-bound phosphines (left and centre).
The new cationic AuPt complex described in this report differs from the existing literature by the presence of weakly bound olefin ligands (in this case, norbonene/nbe) coordinated to the platinum. The other existing examples have only strongly σ-donating phosphine ligands coordinated to the Pt centre, which increases the overall stability of the complexes and renders them unreactive for further chemistry. In contrast, the nbe ligands are more weakly bound, and have a much lower dissociation energy, creating a more reactive complex which is therefore a valuable precursor to other formal Au+IPt0 complexes. This increased reactivity is reflected in the preparation and subsequent manipulations of the complex, which had to be conducted at low temperatures to prevent decomposition.
The new AuPt heterodinuclear complex prepared in this report was characterised by a range of spectroscopic and structural techniques. Single-crystal X-ray diffraction confirmed the molecular structure of the complex, as shown in Figure 2. A rearrangement of the three axial nbe ligands was observed in the new AuPt complex compared to the [Pt(nbe)3] platinum precursor; the three olefin ligands are arranged in a spoke-wheel geometry with the bridging methylenes of nbe all pointing in the same direction away from the gold (an ‘up-up-up’ configuration, in comparison to an ‘up-up-down’ arrangement as in the Pt precursor). NMR spectroscopic characterisation also helped to elucidate and confirm the structure. The 195Pt-NMR resonance of the AuPt complex was particularly noteworthy, showing a similar chemical shift to that of the Pt precursor, which indicates little to no electronic change at Pt0 in the new AuPt complex. This was also reflected in the 13C NMR resonances for the olefinic carbons, which again, were similar in the AuPt complex and the Pt precursor.
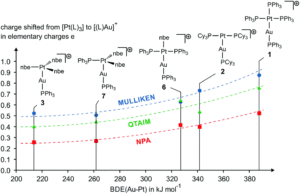
Figure 3: Comparing the new AuPt complex (3) to other systems with fewer bound olefins, in terms of the Au-Pt bond dissociation energy (x-axis) and the overall charge transfer between the Au and Pt (y-axis), according to three different calculations.
The authors then further probed the binding of the gold centre to the platinum, and why there was no apparent significant change in the electronics between the new AuPt complex and the Pt precursor. A comparison to the existing AuPt complexes reported revealed that these are often assigned formally as Au-IPt+II, where there is a dative interaction between the Lewis base (Pt) and the Lewis Acid (Au). In contrast, the new AuPt complex in this report is formally assigned as Au+IPt0, where there is considerably less charge transfer in the metal-metal bonding, as shown by DFT calculations (see Figure 3). This formal Au+IPt0 assignment ultimately results in the coordinated nbe olefin ligands having a low dissociation energy (i.e. they are highly labile and susceptible to ligand substitution), which is further supported by DFT calculations and is reflected in the lack of an identifiable electrospray-ionisation mass spectrometry peak for the [M]+ ion. Therefore, this new AuPt complex is a desirable precursor for the preparation of other formal Au+IPt0 complexes, which will allow for future reactivity studies on these unusual heterodinuclear systems.
To find out more, please read:
A heterodinuclear, formal Au+IPt0 complex with weakly bound alkene ligands
Lukas D. Ernst, Konstantin Koessler, Andreas Peter, Daniel Kratzert, Harald Scherer and Burkhard Butschke
Chem. Commun., 2020, 56, 5350-5353