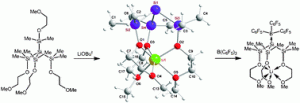
Zwitterionic metal silanides represent a structurally novel class of tri-coordinated silyl anions in which the cationic metal centre is separated from the silicon anion by internal donor bridges.
Clemens Krempner and co-workers have now reported that key to the synthesis of stable, isolable species is the use of pendant polydonor groups that exclusively bind to the metal cation and serve to prevent self-aggregation.
Due to the shape of these unusual compounds, the electron pair located at the central silicon anion is available for additional metal binding. This has allowed for the synthesis of hitherto unknown zwitterionic heterobimetallic silanides, by reaction of the zwitterionic metal silanides with boron, aluminium or tungsten-containing species.
To read about these intriguing compounds in more detail, download the ChemComm communication, which is available for free until April 28th.