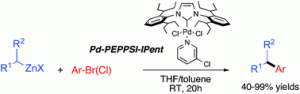
The hugely important field of transition metal catalysed C–C cross-coupling has come a long way over the years, but efficient coupling between Csp2 and secondary Csp3 centres remains a challenge. To achieve just that, Michael Organ and his colleague Selcuk Calimsiz (York University, Toronto) have developed and used a bulky Pd-NHC (N-heterocyclic carbene) catalyst called Pd-PEPPSI-IPent.
In the reactions of secondary alkylzincs with a variety aryl or heteroaryl halides, this catalyst provided excellent regioselectivity for the branched product over the isomerised unbranched product, far outperforming the less bulky Pd-PEPPSI-IPr analogue.
To see the impressive scope of the reaction download the ChemComm communication for free up until 7th March 2011 and leave your comments below.