Zeolitic-imidazolate frameworks (ZIFs) are a sub-class of metal-organic frameworks (MOFs) with a wide range of potential uses including: CO2 capture, storage, catalysis, sensing and biomedicine. Unfortunately their synthesis often requires additives or reaction activation, and if they can be made without these it often requires long reaction times or results in low yields, neither of which is ideal for a substance with such wide potential uses.
To overcome this bottleneck in ZIF synthesis, Roland Fischer and his team from the Inorganic Chemistry department in Ruhr Universitat Bochum in Germany have developed a rapid room temperature synthesis approach. I am a great believer in developing approaches that can be carried out at room temperature and pressure and this is one such elegant solution. The authors produce nanocrystals of ZIFs in a very narrow size distribution by careful selection of the precursors and the solvents they are dissolved in. The solutions are then mixed and stirred to create the ZIF crystals; it really is that elegant.
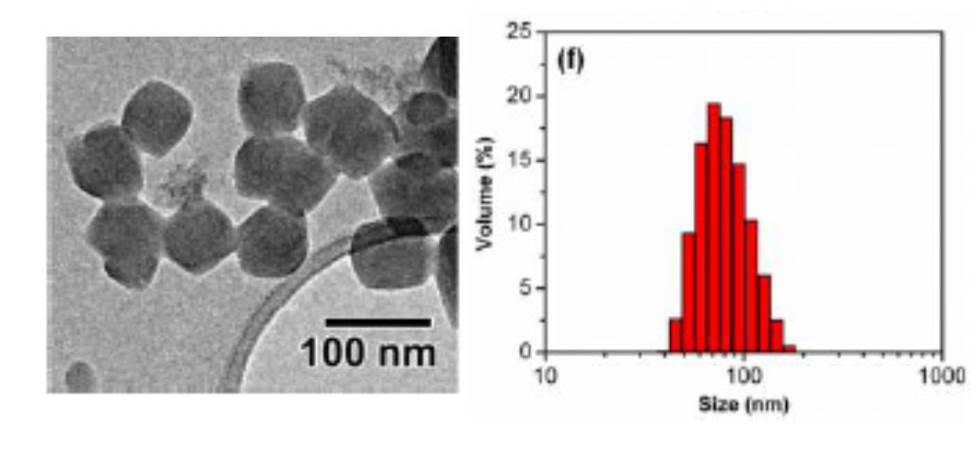
ZIF crystals showing very narrow size distribution
The authors then used these crystals to fabricate thin films on quartz crystal microbalances and used this device to detect volatile organic solvents. This demonstration leads the way into exploring other uses of these ZIFs – after all, they can now be easily made. But to find out which solvent and precursors you need to use, you’ll have to read the paper today!
To read the details, check out the ChemComm article in full:
Rapid room temperature synthesis of zeolitic-imidazolate framework (ZIF) nanocrystals
Min Tu, Christian Wiktor, Christoph Rosler and Roland Fischer
Chem. Commun., 2014, 50, 13258-13260
DOI: 10.1039/C4CC06491G