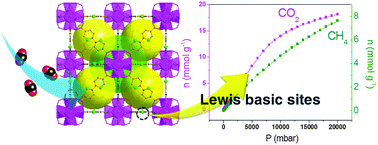
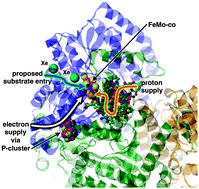
Proteins L1 and L2 form pentamers that arrange to form the viral particle © Shutterstock
Researchers in China have disrupted the life cycle of the leading cause of cervical cancer – the human papilloma virus – using a macrocyclic molecule called a pillarene. The team hope their findings will offer new prophylactic avenues against the virus.There are over 100 different types of the human papilloma virus (HPV), 40 of which can be sexually transmitted. Most infections are symptomless and do not result in disease. However, a few types of the virus are known to cause changes in cells that can lead to cervical and throat cancer. HPV types 16 and 18 cause 70% of cervical cancer cases.
Vaccination programmes against types 16 and 18 have recently become available to teenage girls in some countries. However, as one of the lead scientists on the pillarene project Ying-Wei Yang at Jilin University, China, explains, there is an urgent need for alternatives: ‘the current HPV vaccines are type-specific, expensive and require cold chain transportation, so are not very helpful, especially in developing countries where most cervical cancers occur.’
HPV is made up of two proteins, L1 and L2. These assemble into pentamers to form the virus particles that then attach to cells, resulting in infection. Some researchers believe that disrupting the assembly of the virus using molecules that bind to these two proteins might be the key to stopping it in its tracks.
CP5A, a carboxylatopillar[5]arene sodium salt, has a 3D, rigid and π-rich cavity that binds to amino acids
The pillarene derivative, CP5A, was tested as it is known to have high water solubility and show selective binding towards basic amino acids, like l-Lysine, l-arginine and l-histidine. Because of these properties, CP5A binds to the exposed basic amino acids in protein L1, preventing pentamer formation, and therefore stopping the creation of viral particles.
The team hope to screen other small molecules to find inhibitors for more specific binding sites on the interface between L1 and L2. Their long term aim is to use one of these to produce a HPV vaccine.
Margaret Stanley, a leading expert on the life cycle of human papilloma viruses at the University of Cambridge in the UK sees this study as valuable research for investigations on viral assembly. However, she cautions that the therapeutic value of these approaches is not clear. ‘Inhibiting viral assembly will significantly block transmission, but will not necessarily have any effect on infection level since viral genomes will still be present and potentially able to reactivate after the end of any treatment with inhibitors.’
You can also read this article in Chemistry World»
Read the original journal article in ChemComm – it’s free to download until 28th March:
Efficient inhibition of human papillomavirus 16 L1 pentamer formation by a carboxylatopillarene and a p-sulfonatocalixarene
Dong-Dong Zheng, Ding-Yi Fu, Yuqing Wu, Yu-Long Sun, Li-Li Tan, Ting Zhou, Shi-Qi Ma, Xiao Zha and Ying-Wei Yang
Chem. Commun., 2014, Advance Article, DOI: 10.1039/C3CC49789E