By replacing a single active-site amino acid residue in an aminomutase enzyme, scientists in the Netherlands have engineered a variant that can catalyse the formation of β-tyrosine, an important building block in bioactive compounds.
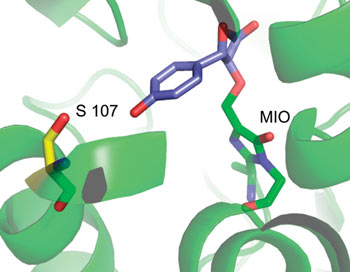
Phenylalanine aminomutase (PAM) is an enzyme that catalyses a chemically challenging α,β-amine shift, forming β-phenylalanine from β-phenylalanine. It can tolerate a wide variety of substituents on the aromatic ring of the substrate but not a para hydroxyl group and so it cannot be used to synthesis β-tyrosine. Although tyrosine aminomutase (TAM) catalyses the amine shift in α-tyrosine to generate β-tyrosine, it has a more limited substrate scope than PAM and has low enantioselectivity towards β-tyrosine.
So Dick Janssen and colleagues replaced a cysteine residue with serine in phenylalanine aminomutase to make an enzyme with both TAM and PAM activity and high enantioselectivity. This engineering enzyme can catalyse the formation of β-tyrosine from p-coumaric acid and may prove useful for synthesising enantiopure β-tyrosine and its derivatives, Janssen says.
For more details, read Janssen’s ChemComm communication, free to access until 12th November. This communication is part of the Enzymes and Proteins web theme issue.