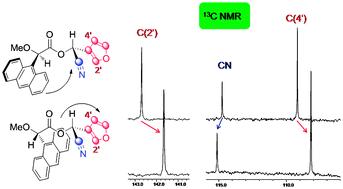
13C NMR shifts can be used, alone or in combination with 1H NMR, to assign the absolute configuration of organic compounds, claim Spanish scientists.
Ricardo Riguera and colleagues at the University of Santiago de Compostela examined the 13C NMR data for a collection of chiral samples, including carboxylic acids and cyanohydrins, derivatised with common auxiliaries. In all cases they found a perfect correlation between the sign of ΔδRS (the difference in chemical shift for the protons around the asymmetric carbon in the R and S substrates) and the absolute configuration of the substrate.
The foundations of the method reside in the aromatic shielding effect produced by the auxiliary on the protons and carbons of the substrate, Riguera explains.
Read the full report in Riguera’s ChemComm communication, free to access until 12th November 2010. And if you enjoy the communication, you might also enjoy NMR methods for unravelling the spectra of complex mixtures, a review in Natural Product Reports by Riguera et al.