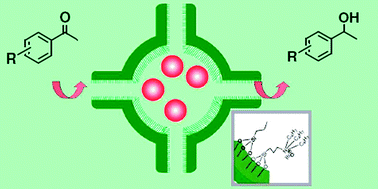
Can Li and his colleagues from Dalian Institute of Chemical Physics, in China, have created a desirable amphiphilic microenvironment within a nanocage that can encapsulate a ruthenium-based catalyst. This nanocage can be ten times more active than one with a hydrophobic environment, where the resulting high catalytic activity can be attributed to the increased ability of the reactants to accumulate inside the nanocage. The team believe that this is mainly due to the enhanced diffusion rates of reactants during the catalytic process.
To read more, why not access the article here, which is free for all to read until the end of August!