If you haven’t heard already, we are speaking to first-time independent researchers who have chosen to publish their first article with ChemComm. This week, we spoke to Amie E. Norton who recently published her #ChemComm1st article: Phase transformation induced mechanochromism in a platinum salt: a tale of two polymorphs.
Find out more from Amie below:
What are the main areas of research in your lab and what motivated you to take this direction?
I work as a Research Chemist at the USDA-ARS. As a Research Chemist at the USDA, I work in the grain quality laboratory. One focus of my research is to synthesize new materials (such as nanomaterials) out of the grain. The motivation to take the research in this direction was that I realized I could use my expertise in a new field, thus merging my Ph.D and the new job I was hired to do. The other focus of my research is to design rapid testing of grain products to measure biochemical components to ensure grain quality. I work under Dr. Michael Tilley to accomplish our research program goals under National Plan 306. Rapid testing of materials to measure biochemical components to ensure grain quality falls under this plan.
Can you set this article in a wider context?
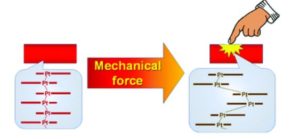
I worked in vapochromic sensors, anion sensors and mechanochromic materials. Our end goal was to design rapid testing methods for anions in drinking water and to have a fundamental understanding on these vapochromic materials. These materials were Pt(II) square planar materials that would change colours when the environment around them changed. They would change colours when introduced to a certain anion or vapor. The complexes were stacking Pt(II) complexes, when the complexes were yellow, they would stack in a dimer or monomer orientation, such as a Pt…Pt…Pt orientation of short…long distances. When the Pt complexes were red, they stacked in a linear chain the Pt…Pt…Pt orientation was short…short distances. The surprising thing is that the Pt(II) complex was often selective in anion sensing to just one anion. The selectivity was built into the complex. I was interested in nitrate as a sensing anion so I decided to play around with adding nitric acid to see if adding a proton helps with the anion exchange with the complex going from yellow to red (understanding the role of pH in the anion exchange). I stumbled on this mechanochromic behaviour and then I grew crystals out of nitric acid (pH 1) and acetone. We went to mount the crystals and they started to change when we touched them with a mounting loop. I knew that structural determination of both of the crystalline forms of mechanochromic material were rare as long range order is often lost in the material after the mechanochromic event. I decided to study this behaviour. It turned out to be one of the most interesting projects, but the discovery of the material was serendipitous.
Describe your journey to becoming independent researcher.
I received a Bachelor’s of Science degree in Chemistry from the University of Missouri (Mizzou). During my time at Mizzou, I studied abroad twice, once in London and once in a two week business class in Prague and Vienna. I also minored in Religious Studies and Sociology; I find that we need to be well-rounded in science. I completed a Ph.D at the University of Cincinnati in 2017 where I worked on vapochromic and anion sensors. My Ph.D taught me how to conduct research, and I mentored 30 undergraduates in the lab setting during this time. I feel it’s important to share knowledge and mentor the next group of future scientists.
Afterwards, I worked at NIOSH (National Institute of Occupational Safety and Health) and learned Real-time Sensing Methods. I helped build a robot out of a Roomba vacuum to survey a room for chemical hazards. Next, I worked as a post-doctoral researcher at Bowling Green State University from 2018-2019 working on photo-active materials. I now work for the USDA-ARS as a Research Chemist (2019-present). Currently, I am working on developing new materials out of grain. My current position has allowed me to learn many aspects of grain research. While I came in with little knowledge of the entire process, I am now able to contribute knowledge from my other areas of study and apply them to my current job. The process of getting grain from the field to our tables is actually a very involved process which I’m proud to be a part of. One thing in my career I have always done is welcome a challenge and take the opportunity to learn new skills as they might be needed in my future work.
What is the best piece of advice you have been given?
There are two pieces of advice that I’ve been given that I always try to follow. My parent’s advice growing up was to have “humble confidence”. The definition of “humble confidence” is to be confident without being arrogant, and to be modest while still projecting competence. The other piece of advice I received was from my Ph.D advisor. He taught me that in research when you see something unusual, most people want to run away from it. Make that unusual more dramatic by designing an experiment to figure out what caused it. Some of the most interesting discoveries are found by the unusual. Do not run away from it, instead become more inquisitive about it.
Why did you choose to publish in ChemComm?
ChemComm has a good reputation. The communication fit well with ChemComm. I felt like the information should be a rapid communication.
#ChemCommMilestones











