Our ChemComm Milestones campaign celebrates new, urgent research from emerging scientists. We recently spoke to Wooseok Ki about this #ChemComm1st article ‘Blue-shifted aggregation-induced enhancement of a Sn(iv) fluoride complex: the role of fluorine in luminescence enhancement‘.
Find out about Wooseok’s experiences as a first-time author in our interview below.
What are the main areas of research in your lab and what motivated you to take this direction?
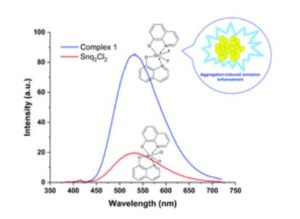
Our primary research goal is to develop and understand the properties of earth-abundant metal based light emitting phosphors using simple solution chemistry. We have developed new tin(IV) halide complex phosphors. Interestingly, our bis(8-hydroquinone)tin(IV) fluoride complex significantly enhances quantum efficiency compared to that of the known, analogous, tin(IV) chloride complex. Furthermore, our tin(IV) flouride complex exhibits interesting aggregation-induced enhancement emission (stronger fluorescence emission in the solid-state than liquid) while the tin(IV) chloride complex does not. Most metal complexes suffer aggregation-induced quenching, weaker emission in the solid-state than liquid, which is a critical issue in OLEDs because OLEDs are fabricated with solid-state film. Therefore, the observed phenomena led to in-depth studies on understanding the role of fluoride ion in the system.
Can you set this article in a wider context?
Most highly efficient metal complexes are composed of expensive rare-earth or noble elements such as Ir, Pt, Re, and Au, which range from 1~90% regarding photoluminescence quantum yield. Despite their excellent performance, one of the drawbacks of using these elements is their high cost elements due to being imported from China. For example, iridium (Ir) costs $41.58 per gram, as reported in 2018, and has been steadily increasing over the years. On the contrary, tin metal is about $0.02/gram. For this reason, abundant, inexpensive transition metal-based complexes have been extensively researched. In our lab, new tin(IV) complexes have been synthesized and characterized by focusing on the effect of halides (i.e., F, Cl, Br, and I) bound to the metal center. In general, the popular way of tuning the optical and electrical properties of metal complexes is to substitute different functional groups in organic molecules(ligands). In our study, we have focused on changing halides bonded with a tin(IV) center with the same organic ligand. Indeed, the choice of halides significantly affects optical, chemical, electrochemical, and structural properties. We are able to tune photoluminescence emission properties systematically. We observed that stronger σ bonding between tin(IV) and fluorine induces significantly improves quantum yield as well as creates aggregation-induced enhancement emission. Our findings would provide to be an important research direction in the way of improving the efficiency of OLEDs.
What do you hope your lab can achieve in the coming year?
In general, the optical emission of metal complexes in the solid-state shows a red-shift with respect to the solution. However, the tin(IV) fluoride complex exhibits blue-shifted aggregation-induced enhancement emission. Therefore, I plan to implement computational studies (Density functional theory) to determine the fundamental mechanism of the fluorinated tin(IV) complex compared with chlorinated tin(IV) complex.
Describe your journey to becoming an independent researcher.
As a materials engineering major, I didn’t explore fundamental chemistry much. My PhD journey allowed me to build up on the fundamental chemistry of inorganic organic hybrid semiconductor materials to understand structure-related properties. After my PhD, I was postdoc at Purdue University and University of Washington, developing earth-abundant thin film solar cells via molecular precursors. Such experiences prepared me as an independent researcher. Furthermore, my industrial experience in Silicon Valley broadened my knowledge and analytical skills, helping to developing my research interests.
What is the best piece of advice you have ever been given?
Failure does not exist in research. Mistakes are stepping stones for new opportunities.
Why did you choose to publish in ChemComm?
ChemComm is a renowned, high-impact journal with fast and excellent support for researchers. The fair review process was the main reason I chose publish in ChemComm.
If you’re interested in reading more outstanding research from first-time authors, head over to our collection ChemComm Milestones – First Independent Articles. You can also find #ChemComm1st related content on our Twitter page: @ChemCommun