Imagine if a pile of laundry could spontaneously organise itself into a neat, folded pile of clothes (and how useful this would be!). This principle can actually happen for some molecules in a process known as self-assembly, whereby a molecule or molecules will organise themselves spontaneously to form a supramolecular structure. Molecules can be specifically designed to self-assemble, by relying on the inclusion of functional groups and motifs that create repulsions and interactions to form unique nanostructures and functional materials.
Researchers in Japan have been studying scissor-shaped azobenzene dyads that can self-assemble by folding, using π-π stacking and hydrogen-bonding (Figure 1a and 1b). They previously observed two key self-assembly processes for two example dyads with formation of either toroidal (doughnut-shaped) structures through intramolecular folding, which can subsequently stack onto each other to create tubular motifs; or fibre/ribbon-type structures through one-dimensional helical stacking. The researchers hypothesised that these different self-assembly pathways could be attributed to differences in the degree of intermolecular aggregation of the dyads and set about verifying this using alkylated or perfluoroalkylated azobenzene dyads (2 and 3, Figure 1a) that have different aggregation properties.
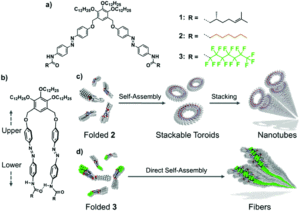
Figure 1. a) Structure of azobenzene dyads 1-3. b) Structure of the folded dyad. (c and d) Self-assembly pathways for dyads 2 (c) and 3 (d).
The researchers prepared the supramolecular assemblies of the new dyads by cooling hot solutions (at 90 °C) of 2 or 3 in methylcyclohexane. Both absorption spectroscopy and dynamic light scattering (DLS) measurements indicated self-assembly of the dyads after cooling to 20 °C, and the resulting assemblies precipitated from the cooled solutions with minutes. The structures of these assemblies were then revealed by atomic force microscopy (AFM), showing different nanostructures for 2 and 3. The alkylated dyad 2 assembled into both toroidal and cylindrically extended nanostructures (Figure 2a and b), where the cylinders were composed of stacked toroidal components (as in Figure 1c). On the other hand, the perfluoroalkylated dyad 3 assembled into entangled fibres after cooling (Figure 2c and d), with no well-defined nanostructures observed even with moderate heating.
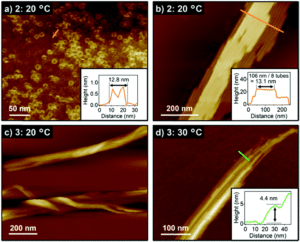
Figure 2. AFM images of the dyad assemblies after cooling to 20 °C. a) toroids of 2 and b) nanotubes of 2, with insets showing cross-sectional analyses along the orange lines. (c and d) supramolecular fibres of 3 [c) cooling to 20 °C and d) cooling to 30 °C], with inset showing cross-section analysis along the green line.
To find out more, please read:
Hironari Arima, Takuho Saito, Takashi Kajitani and Shiki Yagai *
Chem. Commun., 2020, 56, 15619-15622
About the blogger:
 Dr. Samantha Apps recently finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps recently finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.










