Toxic gas removal is essential for preserving the safety of the public and the environment. Examples include gases such as ammonia (NH3) or carbon monoxide (CO) that can be lethal in just parts-per-million concentrations. Typically, removal of these gases is achieved using porous materials with high surface areas such as zeolites, and more recently covalent- or metal-organic frameworks (COFs or MOFs), which soak up the gases by adsorption to sites within the pores of the structure. Ideally such structures would also be recyclable, whereby the gas can be captured and then safely released without damage to the molecular framework of the material. A collaborative effort by researchers across the world has now demonstrated a recyclable strategy for ammonia adsorption by a MOF, using frustrated Lewis pair chemistry to achieve at least 5 cycles of gas capture and release.
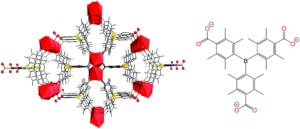
Figure 1. Left: the structure of SION105-Eu, showing its porous nature; right: the chemical structure of the anionic tctb linker ligand (tctb3-)
The researchers selected SION105-Eu as a highly stable MOF, which uses the triply anionic tctb linker that contains a sterically hindered Lewis acidic boron centre (Figure 1). Normally, ammonia (a Lewis base) will interact with a Lewis acidic boron centre to form a Lewis acid-base adduct. However, the steric demands around the Lewis-acidic boron in the tctb linker ligand prevent the formation of a true adduct with ammonia, instead forming a “frustrated Lewis pair” where the ammonia-boron interaction is considered reversible. This notion therefore allows for ammonia capture by adsorption in the MOF through interaction with the boron centre of the ligand, followed by subsequent ammonia release, since a permanent ammonia-boron interaction is prevented by the steric demands of the linker. Additionally, prevention of permanent adsorption of ammonia within the pores of the MOF prevents any possible collapse of the structure/decomposition, which further enhances the recyclability of the material.
The researchers measured the adsorption of ammonia by SION105-Eu by suspending the MOF in an aqueous solution of NH3 at ambient pressures (i.e. with a loose cap on the vial) or high pressures (with a closed vial cap). They observed up to 10 wt% adsorption in the ambient pressure system, and up to 36 wt% adsorption in the closed system (Figure 2, top). The researchers noted retention of the MOF crystallinity upon ammonia adsorption by powder X-ray diffraction measurements (PXRD), where the same pattern was observed before and after (Figure 2, bottom). The PXRD patterns in Figure 2 (bottom) also confirmed the weak, “frustrated Lewis pair” type interactions between the MOF and NH3, by the appearance of only two new peaks as shown in the squared regions of Figure 2 (bottom).
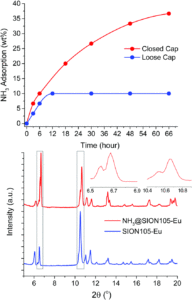
Figure 2. Top: Gravimetrically determined ammonia adsorption over time; bottom: powder XRD patterns before (blue) and after (red) ammonia adsorption
The recyclability of the MOF was tested by first allowing ammonia adsorption by suspension of the MOF in an ammonia solution for 6 hours, followed by ammonia release through heating the saturated MOF at 75 °C for 30 minutes. The researchers demonstrated this process could be achieved 5 times without change to the ammonia adsorption capacity or structure of the MOF. Overall, this study shows a new frustrated Lewis pair strategy for gas capture and release to render a MOF material recyclable, which has the potential to be applied beyond ammonia removal to other toxic gases.
To find out more, please read:
A recyclable metal–organic framework for ammonia vapour adsorption
Tu N. Nguyen, Ian M. Harreschou, Jung-Hoon Lee, Kyriakos C. Stylianou and Douglas W. Stephan
Chem. Commun., 2020, 56, 9600-9603
About the blogger:
 Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.










