Dinitrogen fixation, i.e. converting abundant yet inert nitrogen gas into useable forms, has tantalised chemists for decades. One goal is the transformation of dinitrogen (N2) to ammonia using transition-metal catalysts under acidic and reducing conditions. Just a handful of complexes have been reported so far that can catalyse ammonia formation, and only a few of these are iron based, despite the prevalence of iron in the enzymes that can perform this process biologically. Researchers in the US have now discovered a new iron-based bimetallic system that can catalytically transform N2 to ammonia, providing additional studies that help understand this mechanism (Figure 1).
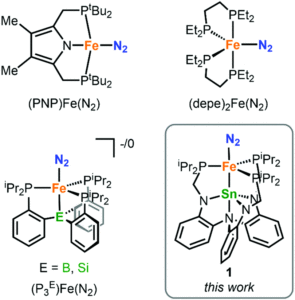
Figure 1: Existing iron systems that can transform N2 to ammonia, and the tin-iron system in this study (bottom right)
The bimetallic system reported by the researchers is a tin-supported iron complex, analogous to other bimetallics previously studied in the group. Dinitrogen complexes were targeted since N2 coordination to a metal centre activates it towards further reactivity. The researchers prepared two tin-iron dinitrogen complexes, by either one or two electron reductions of a metal-bromide precursor (LSnFeBr) under a nitrogen atmosphere, to form the neutral LSnFe(N2) (1) or the anionic [LSnFe(N2)]– (2), respectively. These complexes were characterised using a range of spectroscopic, electronic and structural techniques, which confirmed both coordination of N2 as well as a direct tin-iron interaction (Figure 2).
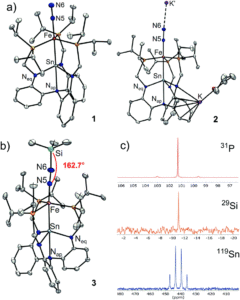
Figure 2. A) X-ray crystal structures of tin-iron dinitrogen complexes 1 and 2. B) Characterisation of the N2 functionalisation product 3 with the crystal structure (left) and NMR spectra (right).
The further-reduced anionic species, 2, showed a greater extent of dinitrogen activation compared to the neutral species (1). This was determined by a lower infrared N-N stretching frequency and a longer N-N bond in the solid-state structure of 2, both of which indicate a weaker and more activated N-N bond. The researchers therefore selected 2 for further functionalisation reactivity, and found that the reaction with Me3SiCl (an electrophile) resulted in successful N2 functionalisation, forming the diazenido complex LSnFeN2SiMe3 (3). The diazenido complex was also characterised using a range of techniques (Figure 2b), with the solid-state structure clearly showing the silyl electrophile adding to the N2 ligand. Isolated diazenido complexes, like 3, are extremely rare in the literature, and the researchers attribute this apparent stability of 3 to the presence of the supporting tin centre within the bimetallic system. The importance of the tin-support in this system was further demonstrated by computational analyses of complexes 1–3, whereby the charge of the distal (and reactive) nitrogen correlated with the charge on the tin centre.
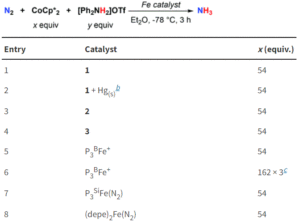
Figure 3: Catalytic ammonia formation from N2 using the tin-iron complexes, with comparison to previously reported iron systems.
The researchers tested all three tin-iron complexes for catalytic ammonia formation, using [Ph2NH2]OTf as an acid and CoCp2* as a reducing agent (Figure 3). Both the neutral dinitrogen complex (1) and the diazenido complex (3) proved successful, with 1 generating up to 5.9 turnovers of ammonia. Additionally, the comparable activity of 3 lends further support to the likely presence of a diazenido intermediate in the mechanism. A comparison with existing iron systems shows that the presence of an iron-support greatly enhances the catalysis compared to unsupported iron (see entry 8, Figure 3), indicating the significance of this metal-support and paving the way towards future catalyst design.
To find out more, please read:
Bimetallic iron–tin catalyst for N2 to NH3 and a silyldiazenido model intermediate
Michael J. Dorantes, James T. Moore, Eckhard Bill, Bernd Mienert and Connie C. Lu
Chem. Commun., 2020, 56, 11030-11033
Samantha Apps acknowledges Michael Dorantes, for proofreading this post, as well as Prof. Connie Lu and the Lu Lab as her colleagues over the last year.
About the blogger:
 Dr. Samantha Apps just finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps just finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.