Micromotors, for the uninitiated aka me, are a specific type of colloidal structure that harvests energy from their environment and turns it into motion. In order for them to be truly effective for possible applications though, they must be able to communicate and coordinate with one another. If you want to make microbots, they can’t just move about all willy-nilly with no regard for each other. Recently, scientists have created a mixed system where one structure sheds silver ions that cause other micromotors to accelerate, an approach that mimics natural systems like bee colonies.
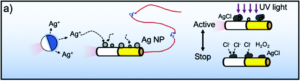
Researchers in China developed a system where photochemically powered micromotors can spontaneously “teach” catalytic micromotors to oscillate without any external influence. The teachers are Janus-type microparticles composed of either polymethylmethacrylate (PMMA) or silicon dioxide particles half coated with silver. Under irradiation with KCl and H2O2, the silver can interconvert between Ag(0) and Ag(1), causing the oscillatory motion of the entire particle. In contrast, the two non-oscillatory micromotors, either polystyrene spheres half coated with platinum (PS-PT) or gold-rhodium microrods (Au-Rh), catalytically decompose H2O2 to move autonomously in standard Brownian motion. When the two types of micromotors are mixed under UV light, the motion of the non-oscillatory materials changes from random to clearly oscillating (Figure 1). The intensity of the motion change depends on the proximity of the learner to the teacher, with learners closer to the teacher displaying more intense oscillations.
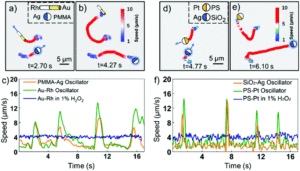
Figure 1. Change in movement of non-oscillating micromotors when exposed to oscillating “teacher” structures.
In fact, the Au-Rh rods will demonstrate more intense oscillations than the PMMA-Ag particles. The researchers propose a mechanism where the PMMA-Ag particles release silver ions as they oscillate, which then deposit onto the Au-Rh rods. The silver increases the catalytic activity of the rods and then, given the operating conditions, undergoes the same redox process that causes oscillation in the PMMA-Ag system.
This hypothesized mechanism is supported by the development of oscillatory behavior by the Au-Rh rods in under reaction conditions where the PMMA-Ag particles are replaced by silver ions in solution. The silver on the rod surface isn’t merely adsorbed – it forms into small silver nanoparticles which can be seen via electron microscopy, making a new trimetallic structure. These nanoparticles change the trajectories of the rods, causing them to move in circles. While this system isn’t perfect, the student structures have imperfect memories and cannot teach one another, it provides a strategy for working with groups of micromotors to move towards coordinated motion and further applications.
To find out more, please read:
Non-oscillatory micromotors ‘‘learn’’ to oscillate on-the-fly from oscillating Ag micromotors
Chao Zhou, Qizhang Wang, Xianglong Lv and Wei Wang
Chem. Commun., 2020, Advance Article
About the blogger:
 Dr. Beth Mundy is a recent PhD in chemistry from the Cossairt lab at the University of Washington in Seattle, Washington. Her research focused on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.
Dr. Beth Mundy is a recent PhD in chemistry from the Cossairt lab at the University of Washington in Seattle, Washington. Her research focused on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.