Piezochromic luminescent materials (PCLMs) exhibit emissive behavior in response to mechanical stimuli and have captured extensive scientific attention, and not just because they’re awesome. Most display reversible bicolor switching, but a lack of universal design principles has hampered development of PCLMs with multiple colors. Utilizing the polymorphs of organic dyes, which generally have different luminescence profiles, is a promising approach towards creating multicolor switches. Iridium (III) complexes can be separately both phosphorescent and polymorphic, but no examples exhibiting both properties had been experimentally shown prior to this work.
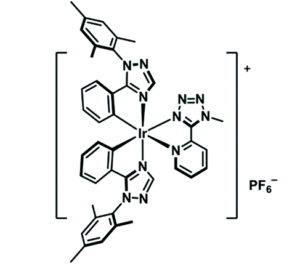
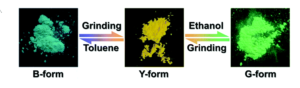
Researchers in China and the UK synthesized a novel cationic Ir(III) complex with a ligand rationally designed for PCL behavior (Figure 1). The bulky ligand should disrupt close crystal packing and leave the material prone to collapse to an amorphous solid when under external pressure. The ligands also consist of heterocycles compounds, which are more likely to have polymorphs, possibly due to the range of non-covalent interactions between different atoms in the solid phase. These cyclic ligands are also quite rigid with limited intramolecular rotational motion, which in studies of aggregates showed that decreasing intramolecular vibrations and rotations can increase luminescence intensity. With all those design considerations put in place, the researchers generated three powder samples of the iridium complex with different emission profiles (blue, yellow, and green). Reproducibly switching the emission color is simple, done by either mechanical grinding or applying solvent.
The initial yellow complex showed low emission when solvated by acetonitrile, but altering the solvent profile to be 90% water induces aggregation and a significant increase in emission. Incorporating the complex into a rigid PMMA matrix similarly enhances its emission, which supports the hypothesis that the rigidity and corresponding diminishment of intramolecular rotations and vibrations serve to enhance the emission. All three solid polymorphs can be obtained directly via recrystallization from different solvents or by cycling through grinding and/or adding small amounts of solvent. Despite the clearly different optical properties, the samples all had identical 1H NMR spectra. The researchers turned to powder X-ray diffraction spectroscopy (PXRD) to gain additional information about the spatial arrangement of the atoms within the molecule and the overall crystallinity of the solids. They saw that the green and blue forms of the complex form distinct crystalline phases, whereas the yellow complex is completely amorphous. The blue crystals show the molecules in antiparallel coupling, while the molecules in the green crystal pack in a herringbone arrangement. However, both pack quite loosely and are thus easily collapsed to an amorphous form when under pressure. Molecular modeling used the obtained molecular confirmations to model the energy levels of the HOMO and LUMO. The models showed that the increased planarity of the phenyltriazole moieties and increase twist of the pyridine-based ligand cause the higher HOMO-LUMO gap, shifting the emission to higher energies.
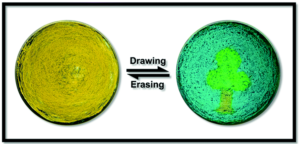
Interestingly, the impact of solvent interactions with the molecules, particularly during crystallization, cannot be discounted. The researchers observed multiple hydrogen-bonding interactions with the iridium complex and either toluene or ethanol. Given the relative ease of switching, as a proof-of-concept the researchers patterned a tree and sky scene starting from the yellow form of the complex and adding ethanol or toluene with a capillary (Figure 3). The scene could be erased and repatterned with no obvious molecular performance degradation.
To find out more, please read:
Reversible tricolour luminescence switching based on a piezochromic iridium(III) complex
Tianzhi Yang, Yue Wang, Xingman Liu, Guangfu Li, Weilong Che, Dongxia Zhu, Zhongmin Su and Martin R. Bryce
Chem. Commun., 2019, 55, 14582-14585.
About the blogger:
 Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.
Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.