A group of scientists from Tsinghua University in China have made a breakthrough in enhancing the controllability of the metal-organic framework (MOF) crystal size.
MOF represents a family of microporous crystals consisting of metal node-organic ligand coordination networks. They have shown potential in versatile applications including hydrogen storage, catalysis and electrochemical energy storage. Since their performance strongly correlates to the crystal size, synthesizing MOF crystals with tunable sizes and high yields is necessary to allow fundamental studies on the size-performance relationship. Unfortunately, the conventional size-controlling methods either require complex operations or exhibit low yields.
Now in ChemComm, Tiefeng Wang and coworkers demonstrate a method that can easily tune the size of MOF crystals. The mechanism is based on decoupling nucleation and growth processes. Unlike traditional strategies that mingle all metal precursors and organic ligands together in a solvent, this newly developed protocol initially mixes only a small portion of metal precursors with organic ligands. The metal precursors quickly coordinate with surrounding ligands to form small MOF clusters (the “nucleation” stage). Due to the limited supply of the metal precursors, the growth of these clusters into large crystals is unfavorable. Subsequently, the remaining metal precursors are introduced into the cluster-containing solution. The clusters then continue to grow into MOF crystals (the “growth” stage). Because the crystals develop directly from the small clusters (i.e. the seeds), the number of the seeds and the total concentration of the added metal precursors control the resulting MOF crystal size (Figure 1).
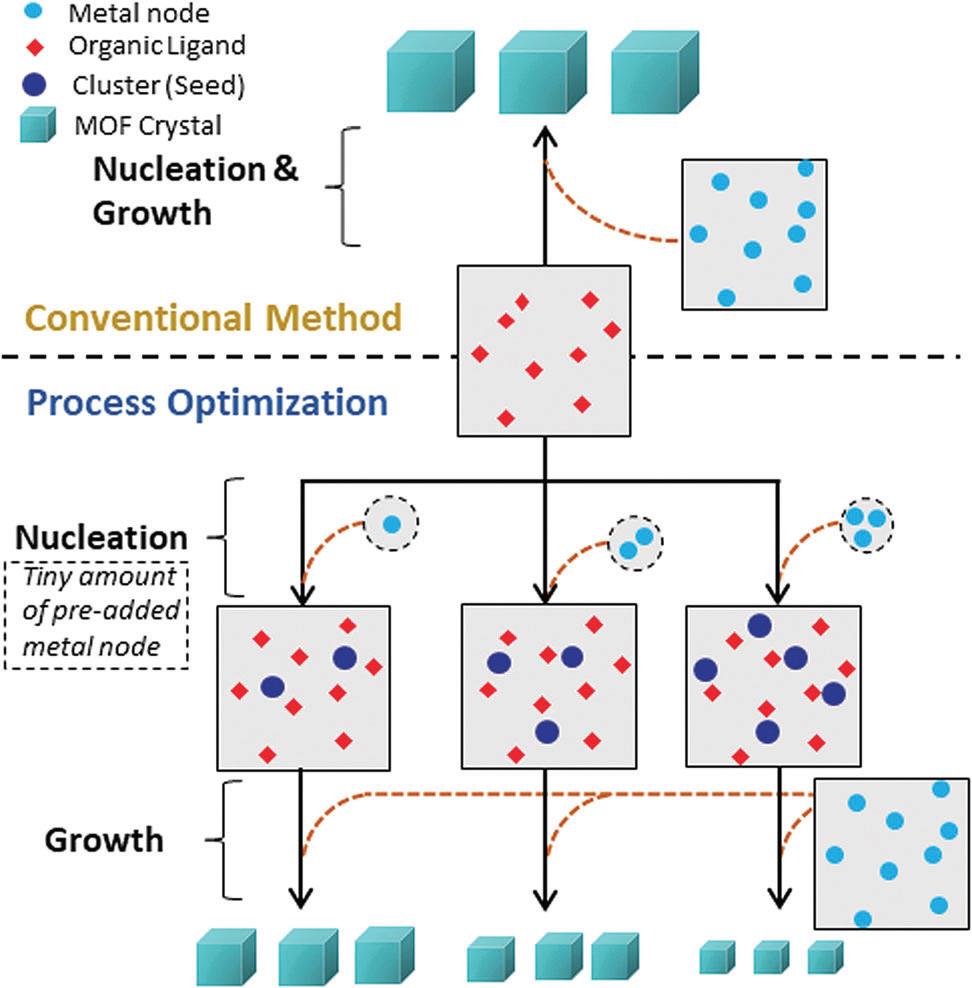
Figure 1. A schematic illustration of the growth of MOF crystals via a typical conventional method (top) and the reported decoupling method (down).
Using this method, the authors prepared a series of Pt@ZIF-8 MOF crystals (with sizes ranging from 45 nm to 440 nm) and investigated their ability to catalyze the reaction of 1-hexene hydrogenation. The catalytic activity of different sized crystals was quantified, with a linear correlation observed between the size and the activity (Figure 2).
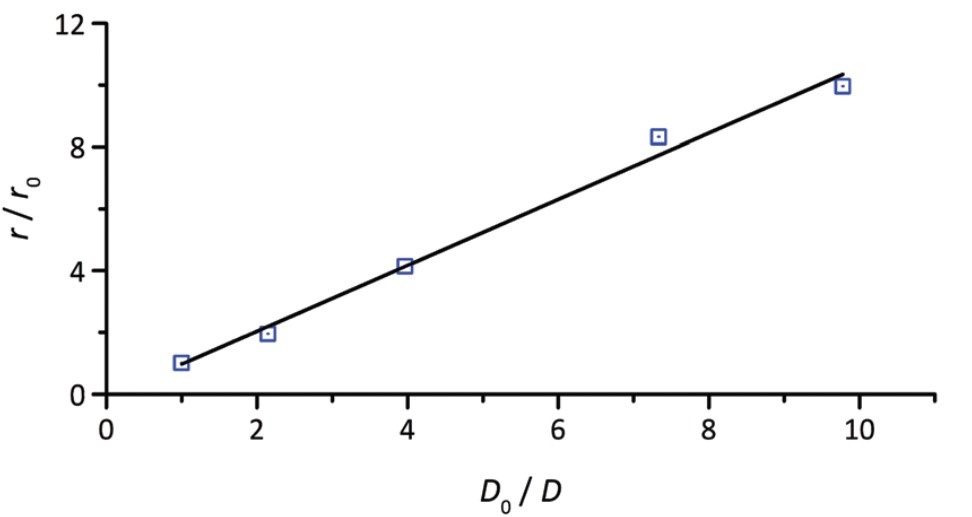
Figure 2. The linear relationship between the Pt@ZIF-8 MOF size (r) and the hydrogenation reaction rate (D). r0 and D0 represent the size and the reaction rate of the smallest MOF (45 nm).
This reported approach is expected to be applicable for synthesizing MOF crystals other than Pt@ZIF-8. The availability of size-tunable MOFs will facilitate mechanistic studies in determining the optimal crystal size for different applications.
To find out more please read:
Xiaocheng Lan, Ning Huang, Jinfu Wang and Tiefeng Wang
Chem. Commun. 2017, DOI: 10.1039/c7cc08244d
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is an online blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is an online blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.










