Metal-organic frameworks (MOFs) are coordination networks consisting of organic ligands and metal cores. They possess crystalline structures with metal complexes as the basic building blocks. These complexes assemble together and extend periodically to form the MOF structures. MOFs represent a family of highly porous materials with ultrahigh surface area (typically >1000 m2 g-1). Other attractive characteristics for MOFs are abundant active metal cores and unique porous structures with tunable pore width, useful for gas storage applications.
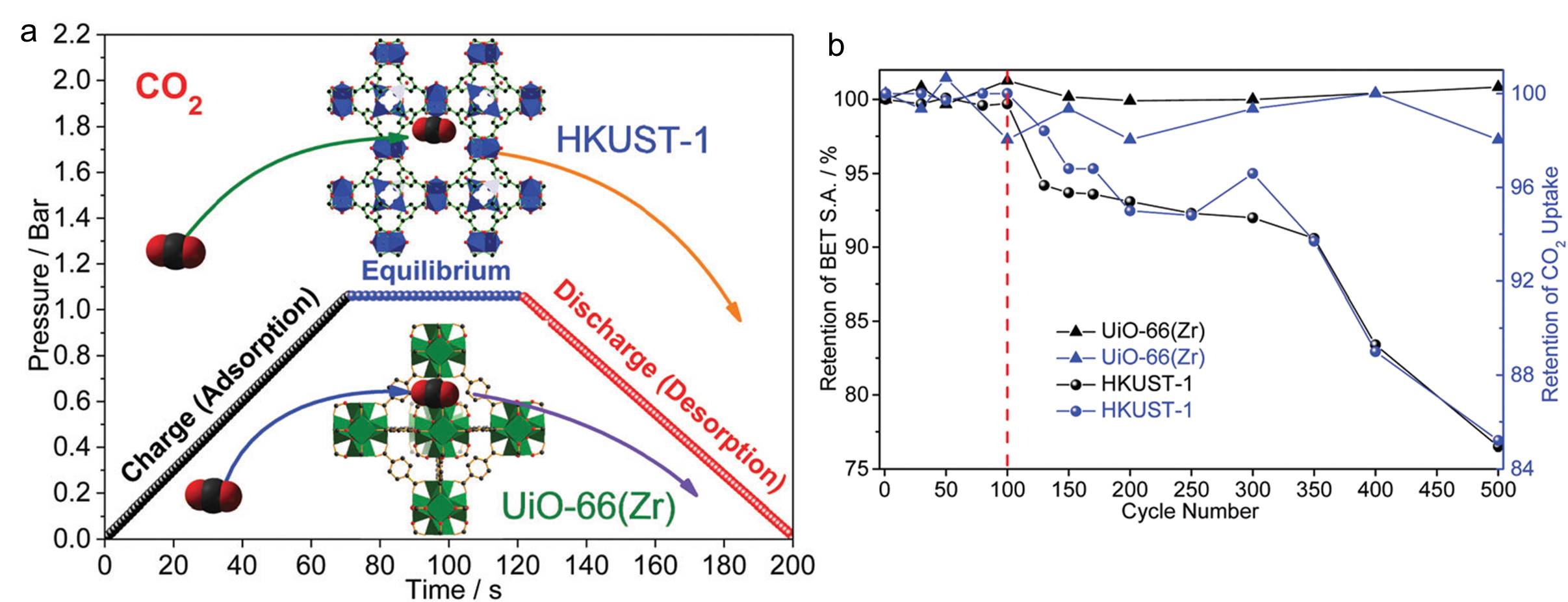
Capturing carbon dioxide has evolved into an intriguing research area, mainly due to environmental concerns triggered by high levels of greenhouse gas emissions. Some MOFs have already been explored as carbon dioxide storage materials and exhibited storage capability exceeding that of conventional absorbents (e.g. amines). Aside from the absorption capacity of carbon dioxide, the performance stability over prolonged operation periods is another figure of merit for MOF-based absorbents. However, there are limited studies in this area. Now for the first time, research groups led by Zeng and Zhao from National University of Singapore compared the performance stability of two representative MOFs, HKUST-1 and UiO-66(Zr). The unit cell of the two MOFs are shown in the inset of Figure a.
The two aforementioned MOFs were subjected to 500 carbon dioxide absorbing and desorbing cycles (Figure a). The carbon dioxide uptake amount of the two MOFs was gauged at specific cycle numbers (Figure b). Whilst HKUST-1 displayed a consistent decreasing storage capacity with increasing cycle number, the capacity of UiO-66(Zr) fluctuated but remained relatively constant. The results clearly indicate that HKUST-1 is more vulnerable and instable than UiO-66(Zr) during long-term working cycles.
The authors then investigated the mechanisms associated with the different stability performances. They first observed that the surface area of HKUST-1 decreased 24% to 1270 m2 g-1 after the stability test, whereas that of UiO-66(Zr) remained relatively intact. Moisture-induced structural collapse was excluded as a possible reason by carrying out a control experiment with ultra-pure and dry hydrogen gas. The authors then exploited multi-frequency atomic force microscopy and concluded that the difference in elastic modulus of the two MOF crystals played an important role in determining the corresponding MOF durability. UiO-66(Zr) has an elastic modulus (ca. 28 GPa) much higher than that of HKUST-1 (ca. 19 GPa), meaning that the former is more elastic than the latter. The high elasticity of UiO-66(Zr) can efficiently buffer the volumetric deformation caused by carbon dioxide absorption and desorption, preventing UiO-66(Zr) crystals from structural failure.
Figure. (a) Illustration of one cycle of the carbon dioxide absorption-desorption test. The inset shows where one carbon dioxide molecule resides in the corresponding MOFs. (b) The evolution of carbon dioxide uptake capacity (blue) and surface area (black) of HKUST-1 and UiO-66(Zr).
This work is expected to provide general guidelines on studying the structural stability of other MOFs with applications associated with gas storage and separation.
To find out more please read:
Structure Failure Resistance of Metal-organic Frameworks toward Multiple-cycle CO2 Sorption
Zhigang Hu, Yao Sun, Kaiyang Zeng, and Dan Zhao
DOI: 10.1039/c7cc04313a
About the author:
 Tianyu Liu is a Ph.D. in chemistry graduated from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu is a Ph.D. in chemistry graduated from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.