Lithium-ion batteries are indispensable for powering a number of electronics (e.g. cell phones, laptops and even electric vehicles) used in the modern society. The key components of a lithium-ion battery are its two electrodes (anode and cathode) because they largely dictate the amount of electrical energy (measured by a parameter called “capacity”) the battery can hold. Increasing the capacity as well as prolonging the lifetime of lithium-ion batteries are practically desirable. Material scientists worldwide are searching for electrode materials to achieve the goal.
In the past decade, a diverse array of metal oxides has been developed as lithium-ion battery anodes with promising performance. These anodes have exhibited considerably higher capacity (~1000 mAh/g) than the current commercial anode material, graphite (372 mAh/g). However, two major drawbacks of metal oxides, namely the limited electrical conductivity and the short lifetime, impede their feasibility for practical applications. While the poor electrical conductivity is an intrinsic physical property of most metal oxides, their short life time is caused by the large volumetric deformation during charge and discharge processes. The deformation will eventually trigger pulverization of electrodes and lead to loss of capacity. Therefore, developing novel strategies that manage to turn metal oxides to viable electrode candidates with satisfying lifetime becomes necessary.
Now writing in Chemical Communications, a research group led by Professor Liqiang Mai and Professor Qi Li from Wuhan University of Technology, China demonstrated a metal oxide-carbon composite anode that exhibited both high capacity and super-long lifetime. The structure of this composite is a “cube-in-tube” configuration (Figure 1): the manganese oxide nanoparticle-embedded carbon “tubes” encapsulate the CoSnO3 (a binary metal oxide) “cubes”. This unique composite electrode delivered a maximal capacity of 960 mAh/g at a current density of 0.1 A/g (Figure 2a), around three times higher than the theoretical capacity of graphite. More impressively, as shown in Figure 2b, the electrode displayed outstanding stability with ~99% of capacity retained after 1500 consecutive charge and discharge cycles (roughly equivalent to four years’ use), much higher than those of the current commercial products and other laboratory-developed composites.
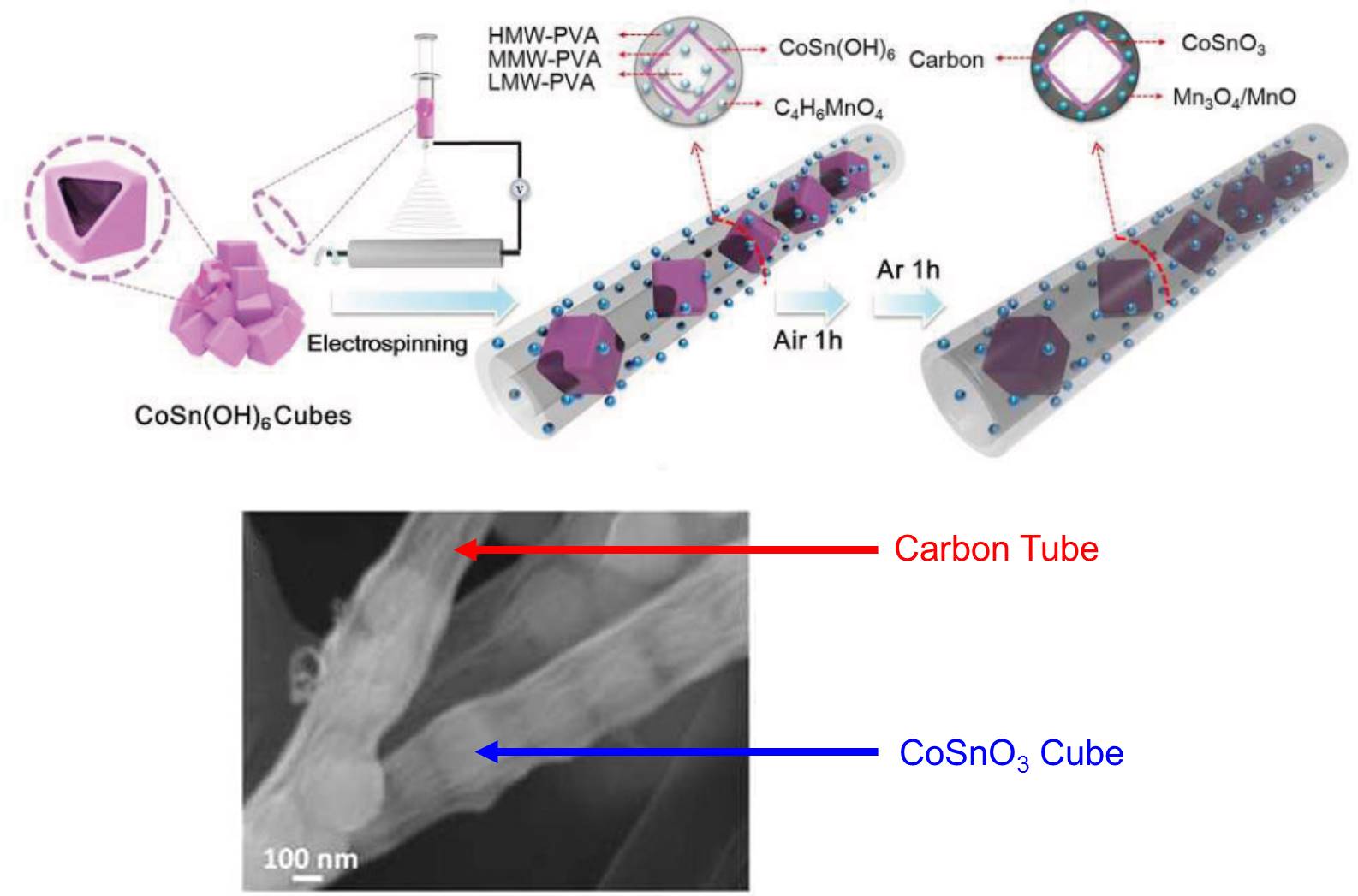 Figure 1. Schematic of the synthesis strategy and the morphology of the “cube-in-tube” metal oxide-carbon composite lithium-ion battery electrode.
Figure 1. Schematic of the synthesis strategy and the morphology of the “cube-in-tube” metal oxide-carbon composite lithium-ion battery electrode.
 Figure 2. (a) Plot of capacity at different current densities; (b) The stability performance evaluated at 2 A/g.
Figure 2. (a) Plot of capacity at different current densities; (b) The stability performance evaluated at 2 A/g.
The authors attributed the electrode’s excellent durability to two reasons. Firstly, the hollow structures (both the “tube” and the “cube”) provide adequate empty space to accommodate volumetric change of metal oxides. Secondly, the soft nature of carbon renders its ability to serve as a mechanical buffer layer. Both aspects reduce the possibility of structural pulverization and promote long lifetime.
To find out more please read:
Facile Electrospinning Formation of Carbon-confined Metal Oxide Cube-in-tube Nanostructures for Stable Lithium Storage
Ziang Liu, Ruiting Guo, Jiashen Meng, Xiong Liu, Xuanpeng Wang, Qi Li, Liqiang Mai
About the author:
Tianyu Liu is a Ph.D. in chemistry graduated from University of California-Santa Cruz, United States. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.










