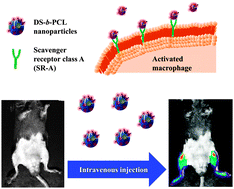
Self-assembled dextran sulphate nanoparticles for targeting rheumatoid arthritis
Seol-Hee Kim, Jong-Ho Kim, Dong Gil You, Gurusamy Saravanakumar, Hong Yeol Yoon, Ki Young Choi, Thavasyappan Thambi, V. G. Deepagan, Dong-Gyu Jo and Jae Hyung Park
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC44260H, Communication
Free to access until 30th September 2013
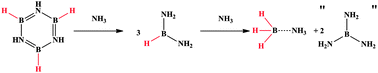
Lewis base assisted B–H bond redistribution in borazine and polyborazylene
Benjamin L. Davis, Brian D. Rekken, Ryszard Michalczyk, Edward B. Garner, III, David A. Dixon, Hassan Kalviri, R. Tom Baker and David L. Thorn
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC44748K, Communication
Free to access until 30th September 2013
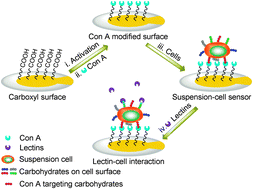
A suspension-cell biosensor for real-time determination of binding kinetics of protein–carbohydrate interactions on cancer cell surfaces
Xueming Li, Yuxin Pei, Ruina Zhang, Qi Shuai, Feng Wang, Teodor Aastrup and Zhichao Pei
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC45006F, Communication
Free to access until 30th September 2013
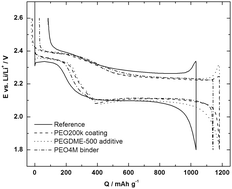
Why PEO as a binder or polymer coating increases capacity in the Li–S system
Matthew J. Lacey, Fabian Jeschull, Kristina Edström and Daniel Brandell
Chem. Commun., 2013,49, 8531-8533
DOI: 10.1039/C3CC44772C, Communication
Free to access until 30th September 2013
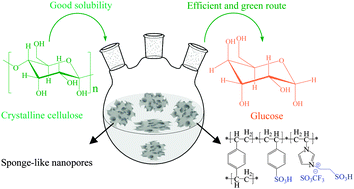
Depolymerization of crystalline cellulose catalyzed by acidic ionic liquids grafted onto sponge-like nanoporous polymers
Fujian Liu, Ranjan K. Kamat, Iman Noshadi, Daniel Peck, Richard S. Parnas, Anmin Zheng, Chenze Qi and Yao Lin
Chem. Commun., 2013,49, 8456-8458
DOI: 10.1039/C3CC44703K, Communication
Free to access until 30th September 2013
Nonvolatile functional molecular liquids
Sukumaran Santhosh Babu and Takashi Nakanishi
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC45192E, Feature Article
Free to access until 30th September 2013
That’s not all– click here for more free HOT Chem Comm articles for August!