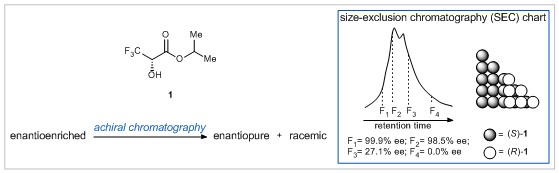
Isopropyl 3,3,3-trifluoro-2-hydroxypropanoate (1) is an important compound for the study of self-disproportionation of enantiomers (SDE), in which an enantiomerically enriched mixture can be separated into enantiopure and racemic portions under achiral conditions. This remarkable separation is made possible by the differences in physicochemical properties of enantiopure and racemic substances. Research led by Professor Vadim A. Soloshonok at the University of the Basque Country has now shed light on the unusual properties of racemic crystals of 1.
True racemic crystals were obtained by sublimation of a mixture of (S)- and (R)- crystal conglomerates at ambient temperature and atmospheric pressure. Surprisingly, when these racemic crystals were analysed, the unit cell was not dimeric in nature as previously thought, but rather contained two distinct (S)- and (R)- enantiomers with no heterochiral H-bonding. The preference of 1 for homochiral intermolecular interactions may explain its extraordinary ability for SDE. Indeed, Soloshonok and co-workers showed that achiral chromatography could be used to obtain enantiopure 1 from an original sample with just 75% ee (see above).
For more, read this ‘HOT’ Chem Comm article today: