Researchers from Leiden University have developed a new method for the synthesis of β–D-rhamnosides (5).
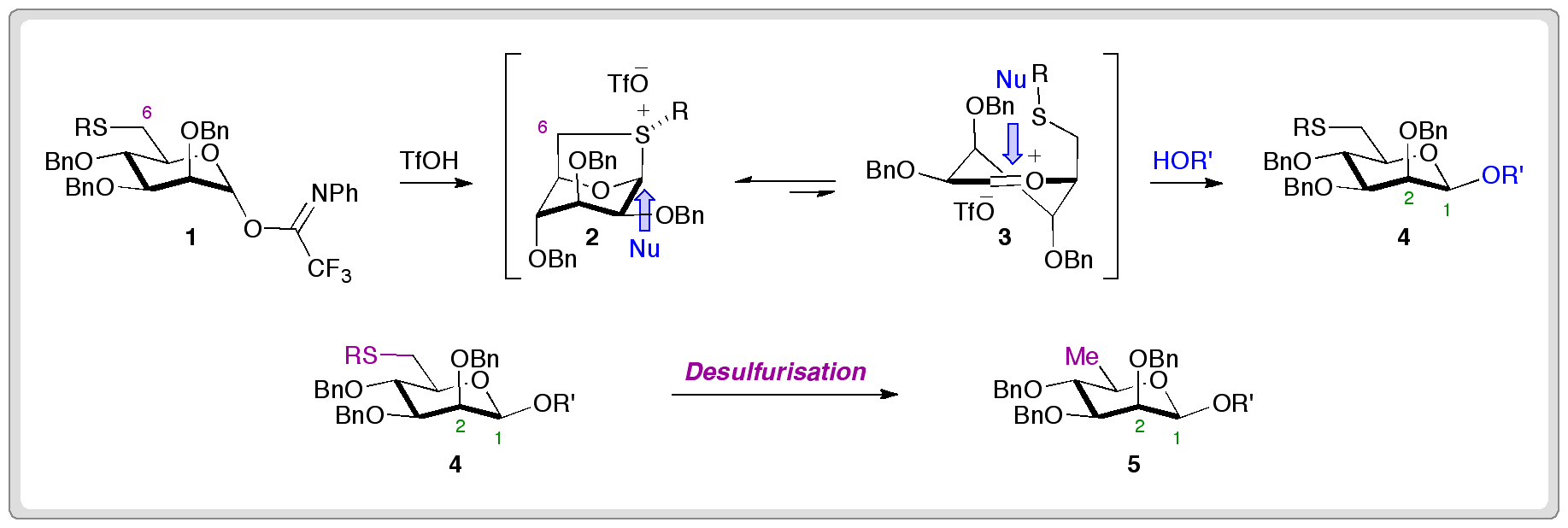
Gijsbert van der Marel’s group showed that C-6 thiophenyl ethers act as stereodirecting groups for condensation reactions of mannosyl donors (1), leading to 1,2-cis products.
They think the reaction proceeds via formation of a bicyclic sulfonium ion (2) that acts as a ‘reservoir’ for a reactive oxocarbenium species (3). Following reaction with an intermolecular nucleophile to form 4, desulfurisation provides the corresponding 1,2-cis-β–D-rhamnoside (5).
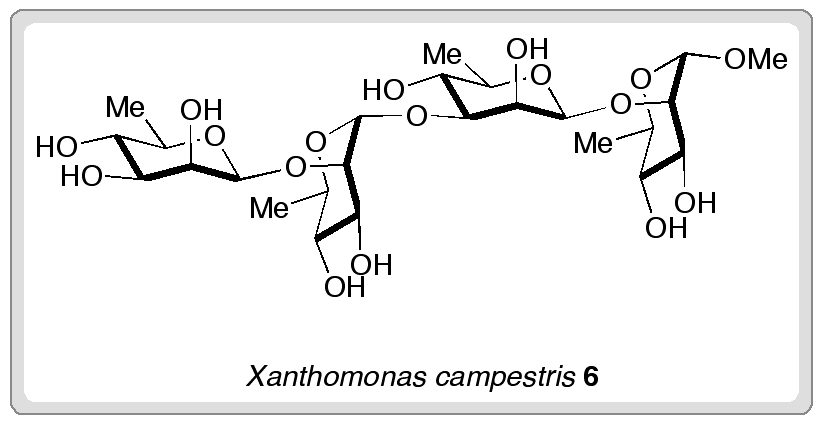
The researchers demonstrated the method’s utility for assembling complex oligosaccharides by making tetrasaccharide 6. This tetrasaccharide forms part of the structure of Xanthomonas campestris pathovar campestris, the causative agent of a devastating disease affecting cruciferous crops such as cabbage and broccoli.
To find out more, download the group’s ChemComm communication.