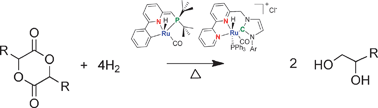
Hydrogenation of esters under mild conditions is difficult at best, however David Milstein and his team push the boundaries by hydrogenating biomass products, glycolide and lactide, to afford the corresponding 1,2-diols. Their synthesis, which utilises ruthenium pincer complex catalysts, offers an atom-economical, green alternative to existing methods for producing 1,2-diols, which currently rely on petroleum-derived ethylene and propylene feedstocks.
The team say that the cyclic di-esters can be selectively and efficiently hydrogenated under very mild conditions, whilst producing no waste, and even discovered that optically pure diols could be produced from chiral lactide.
Download the ChemComm article today to find out how Milstein and his team did this.