New drugs need to be found that are capable of targeting carbonic anhydrases – a class of enzyme that catalyses the hydration of carbon dixoide to bicarbonate and H+. By inhibiting or activating these enzymes, a number of pathological disorders can be treated such as glaucoma, osteoporosis and cancer. Unfortunately, many of the drugs developed so far are not selective for the different isoforms of the enzyme.
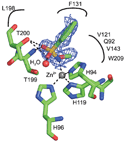
Representation of the binding mode of an inhibitor compound in the active site cavity of the enzyme
Researchers from Italy have embarked upon investigating the inhibition of mammalian isoforms of carbonic anhydrase using N-substituted benzenesulfonamides. By employing X-ray crystallographic studies, they discovered a completely new binding mode with the enzyme. The team say that by substituting the moieties on the phenyl ring, unexplored regions of the enzyme active site could be targeted, allowing new lead compounds to be identified.
Read the ChemComm article to learn more about their findings.