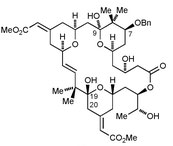
| Bryostatins are macrocyclic natural products with various biological activities, including potent anti-cancer activity due to protein kinase C inhibition. Eric Thomas and his team from the University of Manchester have shown that it is possible to prepare 20-deoxybryostatin, using a modified Julia olefination to form the tricky 16,17-double-bond, followed by macrolactonisation, selective deprotection and oxidation.Fancy finding out more about the reaction conditions?
Download the ChemComm communication, which will be free to access until the 1st July 2011. |
Totally synthetic over bryostatin
03 Jun 2011
UK scientists demonstrate the total synthesis of 20-deoxybryostatin for the first time