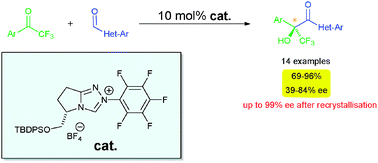
A novel chiral triazolium salt (derived from N-heterocyclic carbene) has shown to be a potent catalyst precursor for the asymmetric cross-benzoin reaction of aldehydes with ketones. Dieter Enders and his colleagues at RWTH Aachen University, in Germany have shown that several heteroaromatic aldehydes can react successfully with various aromatic trifluoroketones in good to excellent yields, with moderate to good enantioselectivities. This could be improved (up to 99% ee) by recrystallisation.
Direct observation of the reaction by NMR along with racemisation experiments showed that the product is formed under kinetic control.
Fancy reading more? Why not download and read Dieter Enders communication today! Its recently been published in ChemComm and will be free to access until the end of September.