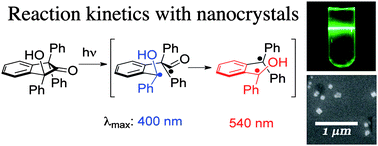
Photochemical reactions in crystals can show excellent selectivity, thanks to the rigid environment. But the development and applications of reactions in crystals has suffered due to a lack of mechanistic insight.
Now Miguel Garcia-Garibay, at the University of California, Los Angeles, US, and colleagues have published the first report addressing the absolute reaction kinetics of a reaction taking place in the crystalline solid state, using conventional pump-probe methods with excitation in the nanosecond and femtosecond time domains.
They studied the Norrish type I decarbonylation of a 2-indanone in solution and as a nanocrystalline suspension and, for the first time, detected the radical intermediates involved in the reaction.
To find out more, download the group’s Chemical Science Edge article for free.
Thinking of submitting an article yourself? No colour charges, no page charges or limits, and free access* providing wide visibility – make Chemical Science your number one choice for your very best research.
* until the end of 2011