A group of scientists led by Prof. Shizhang Qiao has synthesized an oxygen evolution reaction (OER) catalyst combining the merit of low cost, excellent catalytic activity and long lifetime. This OER catalyst is composed of single-crystalline NiFe-hydroxide nanoflakes directly grown on nickel foams. The work has been published recently in ChemComm.
OER, the reaction of producing oxygen gas from water, is an indispensable component of electricity-generation devices using sustainable energy (e.g. fuel cells and photoelectrochemical water splitting cells). OER is usually the bottleneck limiting the overall energy conversion efficiency due to its sluggish kinetics and complex reaction pathways. As such, OER catalysts are needed to accelerate the OER reaction rate. Among the various OER catalysts, noble metal oxides stand out owing to their ultrahigh catalytic activity. However, the “shining” performance is dimmed by their high cost and short lifetime. Thus, obtaining alternatives with comparable OER catalytic activity as well as long-term stability is required to advance the utilisation of sustainable energy.
To address this challenge, the authors turned their attention to a low-cost transition metal, nickel. They developed a hydrothermal method using nickel foams to grow highly crystalline and near-vertically aligned NiFe-hydroxide nanosheets as OER catalysts (Figure 1a). The seamless integration between the hydroxide nanosheets and the nickel substrates reduces the contact resistance and facilitates interfacial electron transfer. The near-vertical orientation (Figure 1b) allows water molecules to fully contact the catalysts. Both of the characteristics render excellent OER catalytic activity. Additionally, the high crystallinity (Figure 1c) ensures the catalysts are robust enough to withstand extensive use without degradation in performance.
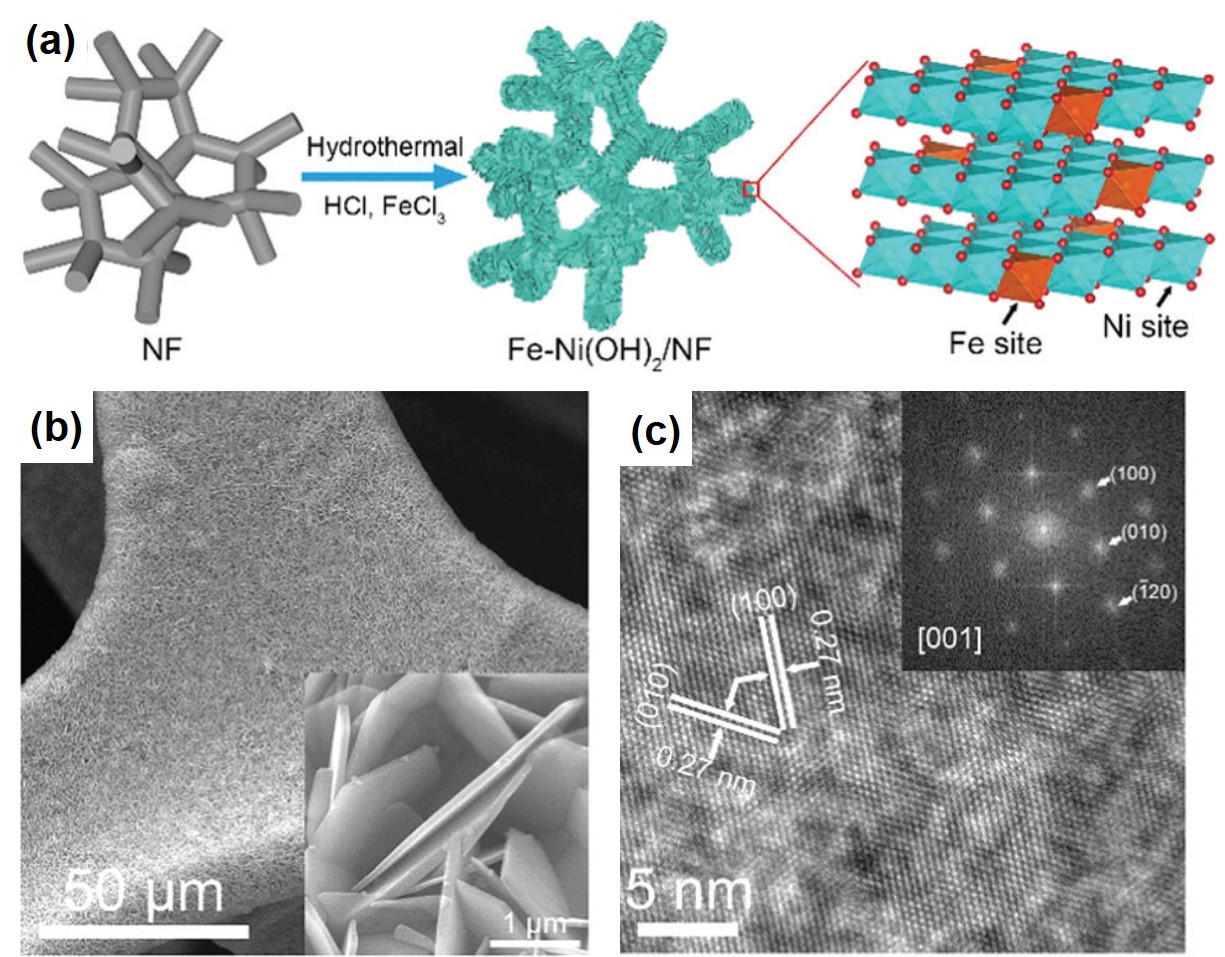
Figure 1. (a) The schematic illustration of the synthetic procedures of the NiFe-hydroxide [Fe-Ni(OH)2] nanosheets supported on nickel foams (NF). (b) The scanning electron microscopy image shows the near-vertically aligned nanosheets on a piece of nickel foam. (c) The transmission electron microscopy image reveals the crystallinity of the synthesized catalyst.

Figure 2. The polarisation curves of different OER catalysts. The onset potential is marked by the dotted line in the inset.
The catalytic activity is also highly stable, with no loss in performance after at least 100 h of measurement. Interestingly, the onset potential further shifts to a lower value of 1.465 V after 100 h. The authors attributed this observation to a “self-activation” process that involves the formation and accumulation of nickel oxyhydroxide (NiOOH) on the surface of the nanosheets.
The hydrothermal method demonstrated here could be used to synthesize other cost-effective crystalline catalysts to develop catalysts for reactions beyond OER, such as hydrogen evolution and carbon dioxide reduction.
To find out more please read:
Jinlong Liu, Yao Zheng, Zhenyu Wang, Zhouguang Lu, Anthony Vasileff and Shi-Zhang Qiao
Chem. Commun. 2017, DOI: 10.1039/c7cc08843d
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.










