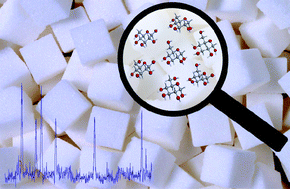
The conformational behaviour of free D-glucose—at last
José L. Alonso, María A. Lozoya, Isabel Peña, Juan C. López, Carlos Cabezas, Santiago Mata and Susana Blanco
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52559G, Edge Article
Free to access until 19th January 2014
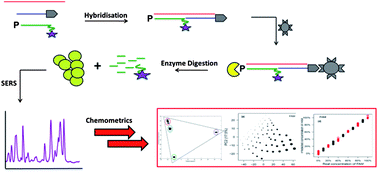
Simultaneous detection and quantification of three bacterial meningitis pathogens by SERS
Kirsten Gracie, Elon Correa, Samuel Mabbott, Jennifer A. Dougan, Duncan Graham, Royston Goodacre and Karen Faulds
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52875H, Edge Article
Free to access until 19th January 2014
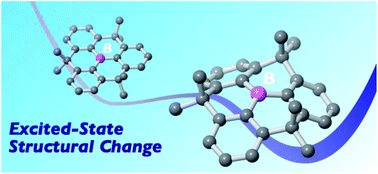
Constraint-induced structural deformation of planarized triphenylboranes in the excited state
Tomokatsu Kushida, Cristopher Camacho, Ayumi Shuto, Stephan Irle, Masayasu Muramatsu, Tetsuro Katayama, Syoji Ito, Yutaka Nagasawa, Hiroshi Miyasaka, Eri Sakuda, Noboru Kitamura, Zhiguo Zhou, Atsushi Wakamiya and Shigehiro Yamaguchi
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52751D, Edge Article
Free to access until 19th January 2014
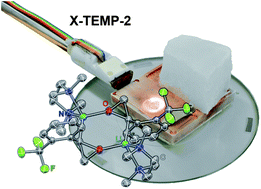
Direct observation of a lithiated oxirane: a synergistic study using spectroscopic, crystallographic, and theoretical methods on the structure and stereodynamics of lithiated ortho-trifluoromethyl styrene oxide
Antonio Salomone, Filippo M. Perna, Aurelia Falcicchio, Sten O. Nilsson Lill, Anna Moliterni, Reent Michel, Saverio Florio, Dietmar Stalke and Vito Capriati
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52099D, Edge Article
Free to access until 19th January 2014
Sugar-coated sensor chip and nanoparticle surfaces for the in vitro enzymatic synthesis of starch-like materials
Ellis C. O’Neill, Abdul M. Rashid, Clare E. M. Stevenson, Anne-Claire Hetru, A. Patrick Gunning, Martin Rejzek, Sergey A. Nepogodiev, Stephen Bornemann, David M. Lawson and Robert A. Field
Chem. Sci., 2014,5, 341-350
DOI: 10.1039/C3SC51829A, Edge Article
Free to access until 19th January 2014
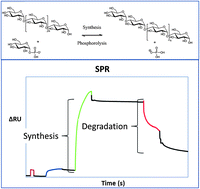
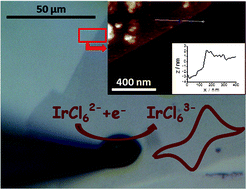
Electrochemistry in a drop: a study of the electrochemical behaviour of mechanically exfoliated graphene on photoresist coated silicon substrate
Peter S. Toth, Anna T. Valota, Matěj Velický, Ian A. Kinloch, Kostya S. Novoselov, Ernie W. Hill and Robert A. W. Dryfe
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52026A, Edge Article
Free to access until 19th January 2014
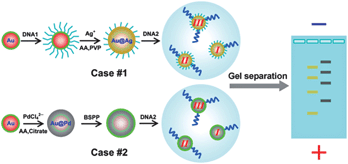
Core solution: a strategy towards gold core/non-gold shell nanoparticles bearing strict DNA-valences for programmable nanoassembly
Huiqiao Wang, Yulin Li, Ming Gong and Zhaoxiang Deng
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52445K, Edge Article
Free to access until 19th January 2014














 Haw Yang is Associate Professor of Chemistry and Director of Graduate Studies at Princeton University, USA. Haw and
Haw Yang is Associate Professor of Chemistry and Director of Graduate Studies at Princeton University, USA. Haw and