We happily breathe our dinitrogen-rich atmosphere all day, but to access nitrogen for the biosynthesis of molecules such as DNA, RNA and proteins, we rely on nitrogen fixation to reduce dinitrogen into bioavailable molecules like ammonia. In nature, nitrogen-fixing bacteria and archaea equipped with nitrogenase enzymes are responsible for providing plants with reduced nitrogen, which makes its way back to us. Nitrogenase is an enzyme complex of two proteins. The first consists of an iron-containing reductase that supplies electrons to the iron/molybdenum-containing catalytic protein, which carries out the N2 to NH3 conversion.
The Haber Bosch process, an industrial method for fixing dinitrogen into ammonia, was first applied in the early 1900’s and generated a huge supply of nitrogen-based fertilizers to synthetically provide plants with this essential nutrient. The population boom that resulted, and with it the global importance of this reaction, has yet to abate. Albeit an efficient reaction, this iron-catalysed process requires high temperatures (450 °C) and pressures (200 atm). In comparison, enzymes can operate under conditions synthetic chemists can only dream of, as researchers at the University of Utah have demonstrated in their work on the bioelectrochemical reduction of dinitrogen under ambient conditions using the catalytic nitrogenase protein.
The researchers synthesised an electrode/enzyme aggregate by trapping the nitrogenase enzyme in a hydrogel, then binding the hydrogel via π-stacking of incorporated pyrene motifs to carbon paper electrodes coated in multi-walled carbon nanotubes. If the enzyme is oriented in the hydrogel in such a way that the distance between the catalytic iron/molybdenum centre of the enzyme and the electrode is within 14 Å, direct electron transfer can take place. Direct electrical contact with enzymes allows researchers to take advantage of the high efficiency and selectivity of enzymes for conducting chemical reactions under mild conditions.
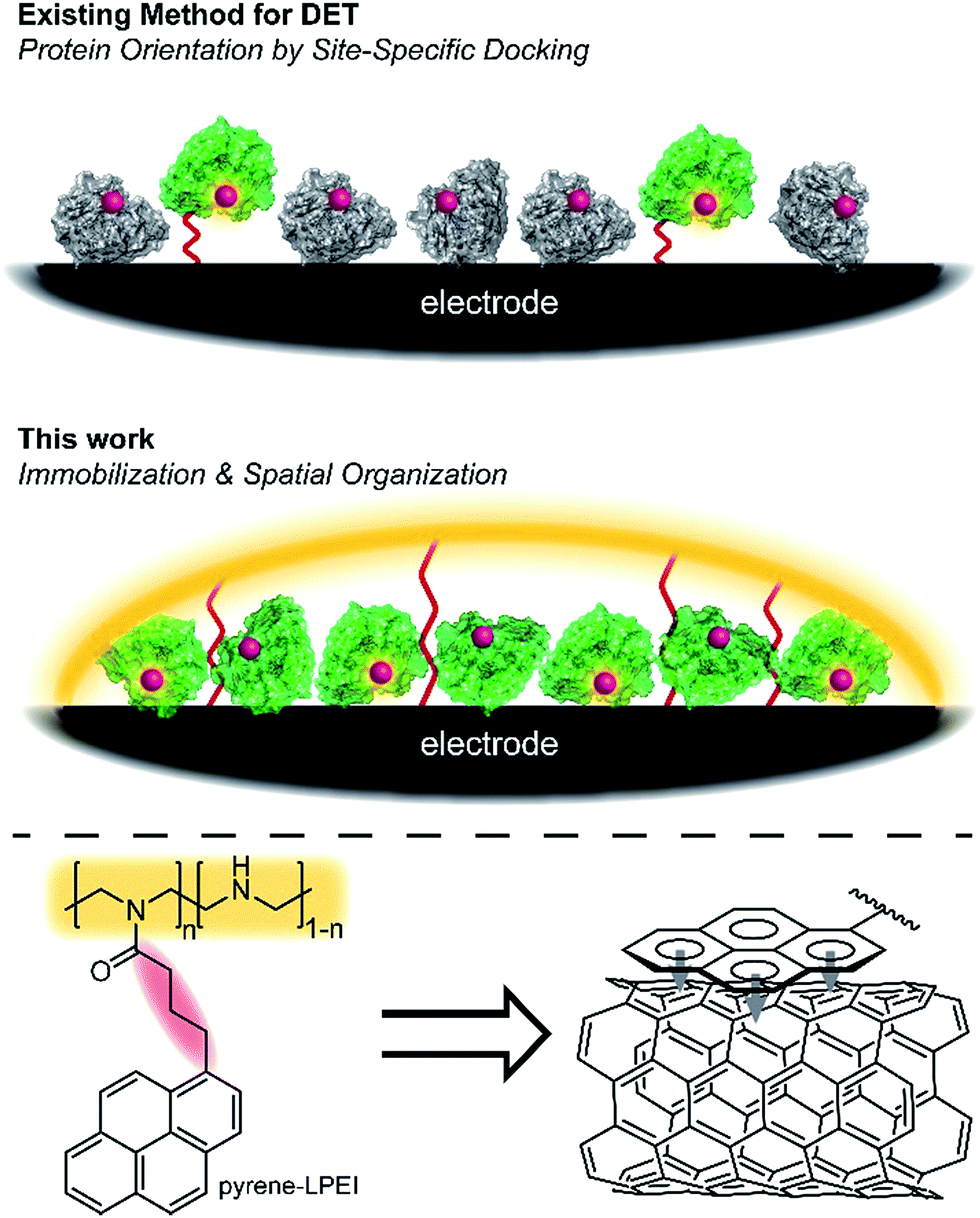
Two methods for immobilization of proteins on an electrode: the docking strategy (upper) and the hydrogel strategy (middle). Active protein is green, while inactive/denatured protein is grey. π-stacking of pyrene moieties within the polymer binds the hydrogel to the carbon nanotubes (lower).
To minimise the distance between the enzyme’s redox centre and the electrode, prior strategies have focused on docking enzymes in the desired configuration; however low enzyme activities can result due to protein denaturation. The authors of this work designed a system under the hypothesis that if they focussed on preserving enzymatic activity, the statistical mixture of configurations adopted by enzymes in the hydrogel would still contain a large proportion capable of participating in direct electron transfer.
Bioelectrical activity of the electrode/nitrogenase aggregate was assessed under bubbling N2 at room temperature, and 180 nmol of NH3 (1.1 μmol/mg nitrogenase enzyme) was produced, marking the first bioelectrochemical reduction of N2 in the absence of ATP. The bioelectrical activity of laccase for the reduction of O2 was also measured using the same method. In this experiment 15% of laccase proteins remained active, compared to 0.3% using a reference method applying an enzyme docking technique. This translated to increased current densities of 390 – 1880 μA cm-2 mg-1 (depending on the enzyme concentration, 1-10 mg mL-1) compared to 45 μA cm-1 mg-1 for the reference docking method.
Without being too grandiose, synthetic nitrogen fixation is vital for the continued survival of people on the planet (how did I do?). Beyond nitrogen fixation, this research offers a general method to achieve contact between a conductive electrode and the highly complex catalytic machinery that nature offers: enzymes. Beyond synthesis, opportunities broaden; technology such as this might pave the way for the production of biosensors, biofuel cells and biomolecular electronic components.
To find out more please read:
Pyrene hydrogel for promoting direct bioelectrochemistry: ATP-independent electroenzymatic reduction of N2
David P. Hickey, Koun Lim, Rong, Cai, Ashlea R. Patterson, Mengwei Yuan, Selmihan Sahin, Sofiene Abdellaoui, Shelley D. Minteer
Chem. Sci., 2018, 9, 5172-5177
DOI: 10.1039/c8sc01638k
 About the author:
About the author:
Zoë Hearne is a PhD candidate in chemistry at McGill University in Montréal, Canada, under the supervision of Professor Chao-Jun Li. She hails from Canberra, Australia, where she completed her undergraduate degree. Her current research focuses on transition metal catalysis to effect novel transformations, and out of the lab she is an enthusiastic chemistry tutor and science communicator.



















 About the author
About the author




 Tianyu Liu is a Ph.D. in chemistry graduated from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web blogger for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at
Tianyu Liu is a Ph.D. in chemistry graduated from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web blogger for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at 
