 Chemical techniques are critical for studying and manipulating biological systems. Here at Chemical Science, we’ve published a host of great articles at the interface of chemistry and biology. Below is a selection of some of the recent ones. Sign up for our e-alerts and browse our issues to keep up-to-date with the latest exceptional research in this fascinating field.
Chemical techniques are critical for studying and manipulating biological systems. Here at Chemical Science, we’ve published a host of great articles at the interface of chemistry and biology. Below is a selection of some of the recent ones. Sign up for our e-alerts and browse our issues to keep up-to-date with the latest exceptional research in this fascinating field.
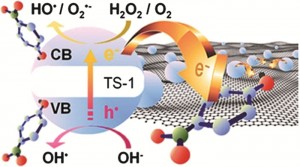
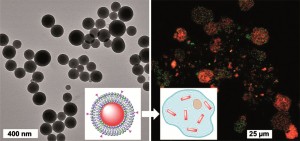
Ultrafast infrared chemical imaging of live cells
Hemmel Amrania, Andrew P. McCrow, Mary R. Matthews, Sergei G. Kazarian, Marina K. Kuimova and Chris C. Phillips, Chem. Sci., 2011, 2, 107-111
Clickable, photoreactive inhibitors to probe the active site microenvironment of fatty acid amide hydrolase
Susanna M. Saario, Michele K. McKinney, Anna E. Speers, Chu Wang and Benjamin F. Cravatt, Chem. Sci., 2011, DOI: 10.1039/C1SC00336D
Molecular recognition of cytochrome c by designed receptors for generation of in vivo and in vitro functions
Satoshi Shinoda and Hiroshi Tsukube, Chem. Sci., 2011, DOI: 10.1039/C1SC00162K
Diversity in natural product families is governed by more than enzyme promiscuity alone: establishing control of the pacidamycin portfolio
Sabine Grüschow, Emma J. Rackham and Rebecca J. M. Goss, Chem. Sci., 2011, 2, 2182-2186
1H NMR metabolomics combined with gene expression analysis for the determination of major metabolic differences between subtypes of breast cell lines
Miroslava Cuperlovic-Culf, Ian C. Chute, Adrian S. Culf, Mohamed Touaibia, Anirban Ghosh, Steve Griffiths, Dan Tulpan, Serge Léger, Anissa Belkaid, Marc E. Surette and Rodney J. Ouellette, Chem. Sci., 2011, 2, 2263-2270
Rapid fluorescence imaging of miRNAs in human cells using templated Staudinger reaction
Katarzyna Gorska, Ioanna Keklikoglou, Ulrich Tschulena and Nicolas Winssinger, Chem. Sci., 2011, 2, 1969-1975
Methods for converting cysteine to dehydroalanine on peptides and proteins
Justin M. Chalker, Smita B. Gunnoo, Omar Boutureira, Stefanie C. Gerstberger, Marta Fernández-González, Gonçalo J. L. Bernardes, Laura Griffin, Hanna Hailu, Christopher J. Schofield and Benjamin G. Davis, Chem. Sci., 2011, 2, 1666-1676
Engineering DNA aptamers for novel analytical and biomedical applications
Mingxu You, Yan Chen, Lu Peng, Da Han, Bincheng Yin, Bangce Ye and Weihong Tan, Chem. Sci., 2011, 2, 1003-1010
Submit your own hot research to our chemical biology and bioorganic Associate Editors: Benjamin Cravatt and Thomas Carrell.


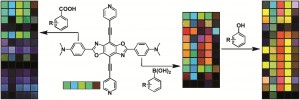










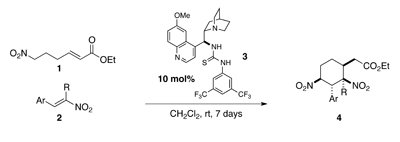




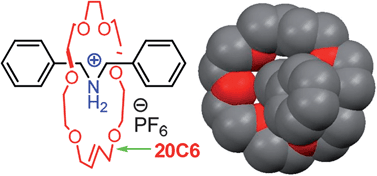 Rotaxanes are dumbbell-shaped molecules that are threaded through a separate macrocylic molecule. The two molecules are not chemically bonded to one another but instead are mechanically interlocked – a feature that can be exploited for molecular switches in molecular electronics, actuators and controlled drug release.
Rotaxanes are dumbbell-shaped molecules that are threaded through a separate macrocylic molecule. The two molecules are not chemically bonded to one another but instead are mechanically interlocked – a feature that can be exploited for molecular switches in molecular electronics, actuators and controlled drug release.