Carbon nanotubes can now be functionalised on a large scale more cleanly and efficiently than before, improving their commercial viability for engineering, catalysis and bionanotechnology.
Milo Shaffer, at Imperial College London, and colleagues, have exploited existing surface oxide defects present in most carbon nanotubes to attach a variety of organic molecules to their surfaces. Surface functionalisation can improve a nanotube’s compatibility with particular environments, such as electrolytes, or can provide a direct function, such as catalytic activity. Conventional functionalisation methods are time-consuming and inconvenient. They also generate a lot of liquid waste, commonly toxic or corrosive. Although some solvent-free methods are known, they are poorly reproducible and often degrade the graphite framework and affect the nanotube’s intrinsic properties.
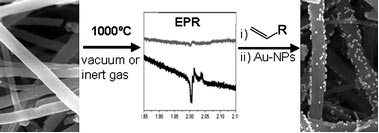
Shaffer heated the nanotubes to 1000 ºC under an inert atmosphere, which caused the defect groups to desorb, leaving reactive surface radicals. When he added functional monomers, such as vinyl compounds, to the activated nanotubes, the monomers polymerised, forming oligomers grafted to the nanotube surface.
This thermochemical method can be applied entirely in the gas phase, which simplifies work-up, improves scalability and makes it compatible with existing gas phase processes for commercially producing nanotubes.
Read the full Chemical Science Edge Article to find out more.