Spongistatin 1 is a highly cytotoxic natural product derived from a marine sponge. In addition to its exquisite biological activity against human cancer cell lines, the structural complexity of spongistatin 1 has captured the imagination of several organic chemistry groups.
A number of total syntheses of the spongistatins exist, but more recently, simplified analogs of the natural products have been tested for anti-cancer activity.
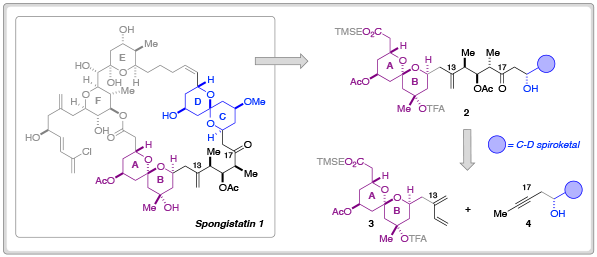
In order to further probe the biological efficacy of simplified spongistatin analogs– or indeed, fragments– researchers at Columbia University have developed new methodology for the synthesis of the A-B fragment 3 and its merger to the C-D spiroketal fragment 3.
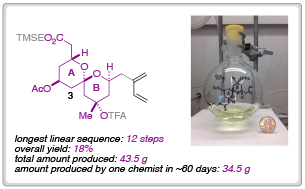
In their most recent Chemical Science Edge article, the Leighton research group have described a synthetic blueprint that could enable the synthesis and testing of a library of ‘C-D spiroketal-modified’ analogs of spongistatin 1. Towards this overarching goal, Samuel Reznik was able to synthesise 34.5 g of fragment 3 in around 60 days; an achievement that demonstrates both the ‘scalability and step-economy’ of this synthetic route.
The Leighton group hope to apply this route to the synthesis of a series of C-D analogs and also hope to develop a complementary synthesis of the E-F fragment to further explore the anti-cancer therapeutic potential of spongistatin 1 derivatives.
For more, read this HOT Chem Sci Edge article in full:
Toward a more step-economical and scalable synthesis of spongistatin 1 to facilitate cancer drug development efforts
Samuel K. Reznik and James L. Leighton
Chem. Sci., 2013, 4, 1497-1501
DOI: 10.1039/C3SC22186E
Alice Williamson is a guest web-writer for Chemical Science. She is currently a postdoc for the OSDDMalaria Project in Dr. Matthew H Todd‘s group at the University of Sydney.












 Read the ‘HOT’ Chemical Science article for free:
Read the ‘HOT’ Chemical Science article for free: