Take a look at this selection of recently published referee-recommended articles – all are open access and free to read:
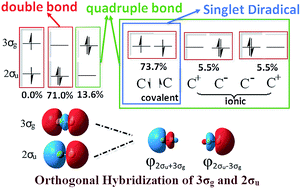
Latent harmony in dicarbon between VB and MO theories through orthogonal hybridization of 3σg and 2σu
Ronglin Zhong, Min Zhang, Hongliang Xu and Zhongmin Su
DOI: 10.1039/C5SC03437J, Edge Article
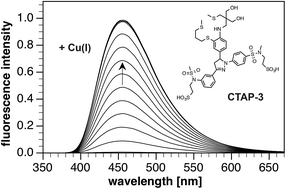
Rational design of a water-soluble, lipid-compatible fluorescent probe for Cu(I) with sub-part-per-trillion sensitivity
M. T. Morgan, A. M. McCallum and C. J. Fahrni
DOI: 10.1039/C5SC03643G, Edge Article
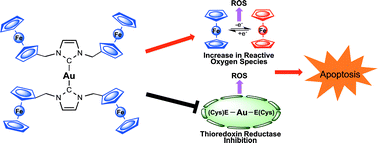
Targeting antioxidant pathways with ferrocenylated N-heterocyclic carbene supported gold(I) complexes in A549 lung cancer cells
J. F. Arambula, R. McCall, K. J. Sidoran, D. Magda, N. A. Mitchell, C. W. Bielawski, V. M. Lynch, J. L. Sessler and K. Arumugam
DOI: 10.1039/C5SC03519H, Edge Article
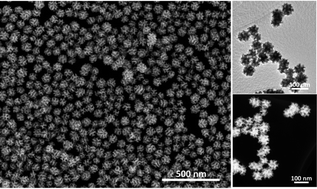
Mesoporous Pt nanospheres with designed pore surface as highly active electrocatalyst
Bo Jiang, Cuiling Li, Victor Malgras, Masataka Imura, Satoshi Tominaka and Yusuke Yamauchi
DOI: 10.1039/C5SC03779D, Edge Article
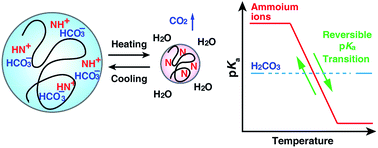
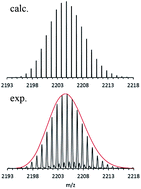
Many Mg–Mg bonds form the core of the Mg16Cp*8Br4K cluster anion: the key to a reassessment of the Grignard reagent (GR) formation process?
T. Kruczyński, F. Henke, M. Neumaier, K. H. Bowen and H. Schnöckel
DOI: 10.1039/C5SC03914B, Edge Article
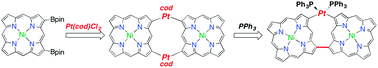
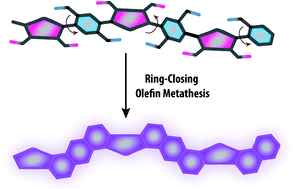
Thermodynamic synthesis of solution processable ladder polymers
Jongbok Lee, Bharath Bangalore Rajeeva, Tianyu Yuan, Zi-Hao Guo, Yen-Hao Lin, Mohammed Al-Hashimi, Yuebing Zheng and Lei Fang
DOI: 10.1039/C5SC02385H, Edge Article
*Access is free through a registered RSC account















 About the Writer:
About the Writer: