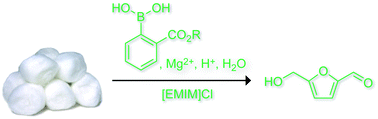
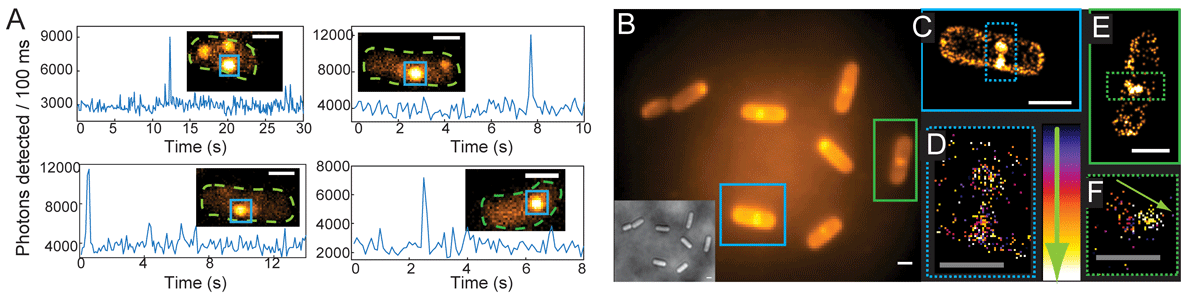
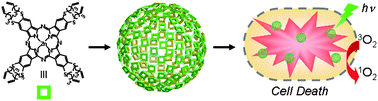
A stimuli-responsive system that brings chemists one step closer to mimicking the complexity of biological networks has been developed by scientists in the UK, Australia and the US.
Biological systems are complicated as they can produce multiple responses to stimuli at the same time. The team says that the key to unravelling the origin of life may come from studying the complex interactions of molecules.
They have discovered a self-assembled cage molecule that consists of a system of interconverting diastereomers in solution. When anionic guest molecules are added, the system adapts, expressing a new combination of diastereomers that synergistically bind the guest molecules. Not only do the cage diastereomers interconvert, the volume of the individual cages adapts physically through the rotation of bonds, providing a tailored binding pocket for the guest lined with hydrogen-bond donors.
This two-fold adaptation is a feature of the responses to external stimuli displayed by biological systems, something that has not previously been observed in synthetic systems. Complex and functional synthetic systems of this type will lead to the design of more effective systems for host-guest recognition and the development of systems approaching the complexity of those that exist in nature.
Read the ‘HOT’ Chemical Science article today:
A Stimuli Responsive System of Self-Assembled Anion-Binding Fe4L68+ Cages
Jack Kay Clegg, Jonathan Cremers, Andrew J Hogben, Boris Breiner, Maarten M. J. Smulders, John D. Thoburn and Jonathan Nitschke
Chem. Sci., 2012, DOI: 10.1039/C2SC21486E