US scientists have synthesised by a new route a key intermediate for the production of synthetic analogues of natural antibiotic tetracyclines that could be used as potential new drugs to combat the growing ranks of antibiotic resistant bacteria.
Andrew Myers and coworkers from Harvard University, Massachusetts, have developed a scalable five step route to an enone intermediate, which can be converted to a range of tetracyclines in three steps. The products are also crystalline at many stages, so there’s no need for purification by chromatography.
The team made the enone by coupling a cyclohexenone with an ester – two inexpensive starting materials made in a few steps from simple precursors. ‘We’ve reduced the problem of tetracycline synthesis to the synthesis of the enone, because from that molecule, you can make completely new tetracyclines,’ says Myers. ‘All tetracyclines that have been approved as drugs in the last 60 years have been made by semi-synthesis – in which fermentation products are used as starting materials – and chemists’ ability to modify these natural products has been limited. We wanted to see if we could develop a completely synthetic route.’
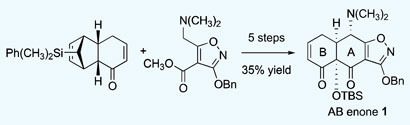
The enone intermediate, a precursor to tetracyclines, was made in five steps by coupling a cyclohexenone with an ester
Myers can now make tetracyclines with modifications all around the structure’s periphery and even in the interior portion. The reaction that transforms the enone into thousands of antibiotics is a Michael-Claisen cyclisation on the left side of the enone, he explains. But it’s also possible to use a similar transformation to modify the right side. ‘Because we’ve got a de novo construction of the enone, we can modify portions of the enone and greatly expand the number of new tetracyclines we can make. In fact, if you think about it, you realise it’s a multiplicative expansion because the expansions on the right side can be coupled with those on the left,’ explains Myers…
To read more, please visit the Chemistry World website or download the Chemical Science Edge Article, which is free to access until the end of 2011!










