Professor Ning Jiao’s Editor’s Choice
We are delighted to share with you our latest Editor’s Choice collection that presents a selection of notable research contributions in synthetic chemistry from 2023 to 2024.
The featured articles selected by Ning demonstrate significant methodological developments such as nitrogen insertion reactions for heterocycle synthesis, machine learning applications for reaction prediction, and innovative bioisostere design for drug discovery.
The collection also highlights advances in organocatalysis, photochemistry, and transition metal catalysis, covering diverse transformations such as C-H functionalization, cycloadditions, and radical reactions. Several studies focus on the synthesis of structurally complex or strained molecules, while others explore sustainable approaches under mild conditions.
These carefully selected works represent rigorous and impactful research, offering valuable insights for synthetic chemists across both fundamental and applied contexts.
Professor Jiao considers work at the forefront of synthetic chemistry, including significant advances in catalysis and organic methodology. Submit your best manuscripts on these topics to Chemical Science for Professor Jiao’s consideration.
We hope you enjoy reading this selection of articles selected by Professor Ning Jiao.
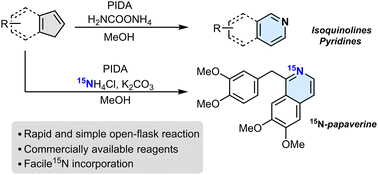
Nitrogen atom insertion into indenes to access isoquinolines
Patrick Finkelstein, Julia C. Reisenbauer, Bence B. Botlik, Ori Green, Andri Florin, Bill Morandi
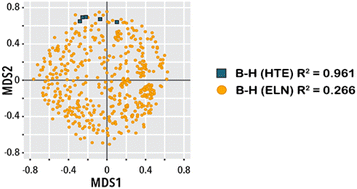
On the use of real-world datasets for reaction yield prediction
Mandana Saebi, Bozhao Nan, John E. E. Herr, Jessica Wahlers, Zhichun Guo, Andrzej M. M. Zuranski, Thierry Kogej, Per-Ola Norrby, Abigail G. G. Doyle, Nitesh V. V. Chawla, Olaf Wiest
1,2-Disubstituted bicyclo[2.1.1]hexanes as saturated bioisosteres of ortho-substituted benzene
Aleksandr Denisenko, Pavel Garbuz, Yelyzaveta Makovetska, Oleh Shablykin, Dmytro Lesyk, Galeb Al-Maali, Rodion Korzh, Iryna V. Sadkova, Pavel K. Mykhailiuk
In situ polymerization of 1,3-dioxolane and formation of fluorine/boron-rich interfaces enabled by film-forming additives for long-life lithium metal batteries
Ting Li, Kai Chen, Borui Yang, Kun Li, Bin Li, Miao He, Liu Yang, Anjun Hu, Jianping Long
Synthesis of polysubstituted bicyclo[2.1.1]hexanes enabling access to new chemical space
Marius Reinhold, Justin Steinebach, Christopher Golz, Johannes C. L. Walker
Lewis acid-catalyzed diastereoselective carbofunctionalization of bicyclobutanes employing naphthols
Avishek Guin, Subrata Bhattacharjee, Mahesh Singh Harariya, Akkattu T. Biju
Cobalt-catalyzed enantioselective C-H/N-H annulation of aryl sulfonamides with allenes or alkynes: facile access to C-N axially chiral sultams
Xiao-Ju Si, Xiaofang Zhao, Jianli Wang, Xinhai Wang, Yuanshuo Zhang, Dandan Yang, Mao-Ping Song, Jun-Long Niu
Lemniscular carbon nanohoops with contiguous conjugation from planar chiral [2.2]paracyclophane: influence of the regioselective synthesis on topological chirality
Jing He, Mo-Han Yu, Zhe Lian, Yan-Qing Fan, Sheng-Zhu Guo, Xiao-Nan Li, Ying Wang, Wen-Guang Wang, Zhi-Yun Cheng, Hua Jiang
Triarylamines as catalytic donors in light-mediated electron donor-acceptor complexes
Durbis J. Castillo-Pazos, Juan D. Lasso, Ehsan Hamzehpoor, Jorge Ramos-Sanchez, Jan Michael Salgado, Gonzalo Cosa, Dmytro F. Perepichka, Chao-Jun Li
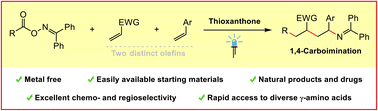
Metal-free photosensitized radical relay 1,4-carboimination across two distinct olefins
Guangying Tan, Fritz Paulus, Alessia Petti, Maxim-Aleksa Wiethoff, Anna Lauer, Constantin Daniliuc, Frank Glorius
2,5-disubstituted bicyclo[2.1.1]hexanes as rigidified cyclopentane variants
Shashwati Paul, Daniel Adelfinsky, Christophe Salome, Thomas Fessard, M. Kevin Brown
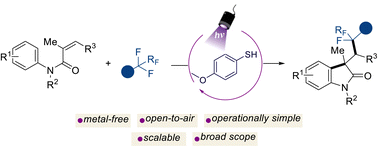
Transition metal-free photochemical C-F activation for the preparation of difluorinated-oxindole derivatives
Bianca Matsuo, Jadab Majhi, Albert Granados, Mohammed Sharique, Robert T. Martin, Osvaldo Gutierrez, Gary A. Molander
Stereoselective alkyl C-glycosylation of glycosyl esters via anomeric C-O bond homolysis: efficient access to C-glycosyl amino acids and C-glycosyl peptides
Anrong Chen, Shiyin Zhao, Yang Han, Zhenghong Zhou, Bo Yang, Lan-Gui Xie, Maciej A. Walczak, Feng Zhu
Lewis acid catalyzed [4+2] annulation of bicyclobutanes with dienol ethers for the synthesis of bicyclo[4.1.1]octanes
Stefano Nicolai, Jerome Waser
Palladium-catalyzed decarboxylative (4+3) cycloadditions of bicyclobutanes with 2-alkylidenetrimethylene carbonates for the synthesis of 2-oxabicyclo[4.1.1]octanes
Xin-Yu Gao, Lei Tang, Xu Zhang, Jian-Jun Feng
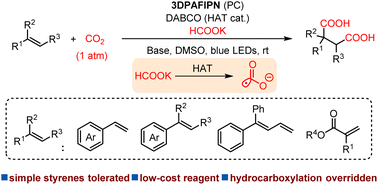
Visible-light-driven alkene dicarboxylation with formate and CO2 under mild conditions
Fulin Zhang, Xiao-Yang Wu, Pan-Pan Gao, Hao Zhang, Zhu Li, Shangde Ai, Gang Li
One-pot asymmetric living copolymerization-induced chiral self-assemblies and circularly polarized luminescence
Run-Tan Gao, Shi-Yi Li, Bing-Hao Liu, Zheng Chen, Na Liu, Li Zhou, Zong-Quan Wu
De novo synthesis of inherently chiral heteracalix[4]aromatics from enantioselective macrocyclization enabled by chiral phosphoric acid-catalyzed intramolecular SNAr reaction
Xing-Chi Li, Ying Cheng, Xu-Dong Wang, Shuo Tong, Mei-Xiang Wang
If you are interested in similar areas, explore our most popular organic and catalysis themed collections!
Chemical Science is the flagship journal of the Royal Society of Chemistry, publishing exceptional research across the chemical sciences. As a diamond open access journal, all of our articles are free to read and free to publish – find out more and browse our latest articles on our webpage.
Keep up to date with our latest articles, reviews, collections & more by following us on Bluesky, LinkedIn or by signing up to our E-Alerts.



![Graphical abstract: 1,2-Disubstituted bicyclo[2.1.1]hexanes as saturated bioisosteres of ortho-substituted benzene](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3SC05121H&imageInfo.ImageIdentifier.Year=2023)

![Graphical abstract: Synthesis of polysubstituted bicyclo[2.1.1]hexanes enabling access to new chemical space](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3SC03083K&imageInfo.ImageIdentifier.Year=2023)


![Graphical abstract: Lemniscular carbon nanohoops with contiguous conjugation from planar chiral [2.2]paracyclophane: influence of the regioselective synthesis on topological chirality](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D2SC06825G&imageInfo.ImageIdentifier.Year=2023)

![Graphical abstract: 2,5-disubstituted bicyclo[2.1.1]hexanes as rigidified cyclopentane variants](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3SC02695G&imageInfo.ImageIdentifier.Year=2023)

![Graphical abstract: Lewis acid catalyzed [4+2] annulation of bicyclobutanes with dienol ethers for the synthesis of bicyclo[4.1.1]octanes](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D4SC02767A&imageInfo.ImageIdentifier.Year=2024)
![Graphical abstract: Palladium-catalyzed decarboxylative (4 + 3) cycloadditions of bicyclobutanes with 2-alkylidenetrimethylene carbonates for the synthesis of 2-oxabicyclo[4.1.1]octanes](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D4SC02998D&imageInfo.ImageIdentifier.Year=2024)

![Graphical abstract: De novo synthesis of inherently chiral heteracalix[4]aromatics from enantioselective macrocyclization enabled by chiral phosphoric acid-catalyzed intramolecular SNAr reaction](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3SC06436K&imageInfo.ImageIdentifier.Year=2024)








