Organoboranes are extremely useful reagents for chemical synthesis; their Lewis acidic nature makes them reactive towards nucleophilic species, and their ability to participate in free-radical processes widely expands their synthetic use. Trialkylboranes (BR3) are the most widely studied in terms of borane radical chemistry, whereby alkyl radicals (R●) can be generated through homolytic substitution at the boron atom under oxygen conditions to then participate in various alkylation reactions, as shown in Scheme 1a. This extremely mild radical-initiation system, using just O2 instead of heat or light for radical generation, is highly desirable in chemical synthesis, particularly for the formation of thermally unstable products.
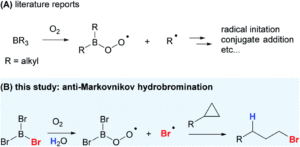
Scheme 1: (A) Previously known radical chemistry with organoboranes and (B) radical reactivity using trihaloboranes.
Researchers in both China and the US have now applied this concept of radical generation using trihaloboranes for halogenation. Halogenation reactions are extremely important in chemical synthesis, since the resulting halogenated products are ideal precursors for installing a wide range of functional groups through substitution chemistry. Typically, halogenation of organic molecules using trihaloboranes has been attributed to their Lewis acidic nature, but the researchers have now shown that these reagents can also act as halogen radical donors (as shown in Scheme 1b).
The researchers selected boron tribromide (BBr3) as a bromide radical donor (Br●), since the B-O bond that forms upon radical generation using O2 is much stronger than the B-Br bond that breaks, making the process thermodynamically favourable. They applied this approach to investigate the hydrobromination of cyclopropanes, for the novel and selective formation of the anti-Markovnikov haloalkane product. The researchers initially optimised the reaction of cyclopropylbenzene (1a) with BBr3/O2 and found that the addition of a proton source (e.g. H2O or alcohol) was sufficient to terminate the radical reaction and give the anti-Markovnikov product (2a) as the major species (Scheme 2). Using these conditions, the substrate scope could be expanded for the hydrobromination of a wide range of cyclopropanes, including typically challenging alcohol or amine-functionalised substrates.
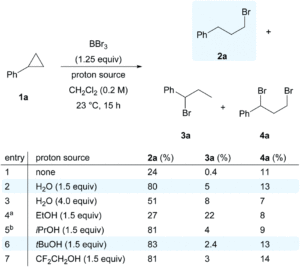
Scheme 2: Initial reaction optimisation of hydrobromination of cyclopropylbenzene (1a) to give the anti-Markovnikov product (2a) as the major species.
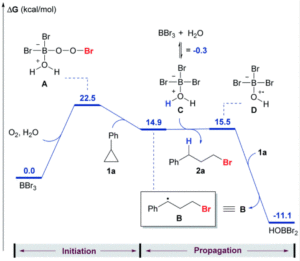
To establish that the hydrobromination reactivity was occurring via a radical process rather than a possible acid-mediated pathway, the researchers conducted a series of control experiments. The addition of radical scavengers resulted in only the formation of the Markovnikov product, suggesting the radical process is necessary for the anti-Markovnikov selectivity observed. The absence of oxygen also shut down the reactivity, which further indicates the radical pathway as shown in Scheme 1b. Additional computations modelled a possible pathway analogous to the established radical alkylation using trialkylboranes, showing an energetically favourable radical pathway for the hydrobromination of cyclopropylbenzene using BBr3/O2 (Figure 1).
The results in this study demonstrate that trihaloboranes, like trialkylboranes, can act as radical donors for halogenation reactions, allowing for previously unreported anti-Markovnikov selectivity in the hydrobromination of cyclopropanes. This radical reactivity could be applied in the future for the halogenation of many different organic molecules, giving way to new methods to affect selectivity that cannot be achieved using traditional acid-mediated pathways.
To find out more, please read:
Boron tribromide as a reagent for anti-Markovnikov addition of HBr to cyclopropanes
Matthew H. Gieuw, Shuming Chen, Zhihai Ke, K. N. Houk and Ying-Yeung Yeung
Chem. Sci., 2020, 11, 9426-9433
About the blogger:
 Dr. Samantha Apps just finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps just finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.