Fluorescence, the phenomenon where a molecule re-emits light upon absorption of electromagnetic radiation, is used in biological imaging to visualise structures, processes and diseases. Emission of these fluorescent molecules, known as fluorophores, in the near-infrared region is particularly advantageous, allowing for enhanced tissue penetration and reduced photodamage. Near-infrared (NIR) fluorophores are therefore attractive probes for bioimaging but are currently limited with problems such as low brightness or quenching of the emission by aggregation.
To overcome this aggregation-caused quenching effect, researchers in China turned to fluorophores that have aggregation-induced emission (AIE) properties. Aggregation-induced emission (AIE) is a concept where molecules only fluoresce upon aggregation in concentrated solutions, and not in dilute solutions where they can freely rotate. The researchers therefore designed their fluorophore to contain the molecular rotor tetraphenylethene, that can induce AIE effects and therefore boost and brighten the fluorescence.
The researchers prepared a suite of fluorophores using a central donor-acceptor-donor core, with methoxy-tetraphenylethene (MTPE) as the donor and thieno[3,4,-b]pyrazine (TP) as the acceptor. Substituents on the TP acceptor were varied, and the effects on aggregation and the fluorescence were investigated. Density functional theory calculations gave the researchers insight into the molecular conformations of the fluorophores, as shown in Figure 1. The 3 variants all showed twisted geometries (top row, Figure 1), indicating high degrees of rotation, which could then be restricted through aggregation and give rise to the desired AIE effects. Additionally, the calculations measured electronic distributions, confirming high degrees of electron conjugation in the molecules (see the HOMO diagrams, Figure 1) that are essential for fluorescence.
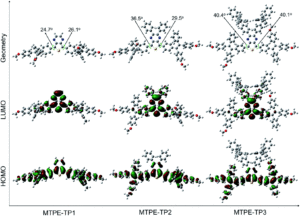
Figure 1: Results from density functional theory calculations to show molecular geometries and electron conjugation within the suite of fluorophores
The fluorescence characteristics of the variants were measured by absorption and emission/photoluminescence spectra. The absorption spectra in DMSO (Figure 2a) shows absorptions between 518 and 543 nm, with the most red-shifted (longer wavelength) absorption displayed for the most conjugated variant (MTPE-TP3). The effect of aggregation on the fluorescence was measured by adding water (in which the fluorophores showed poor solubility) to the DMSO solutions, and the resulting photoluminescence intensities showed an increase with higher water fractions. This increase in brightness (i.e. intensity) is explained by the water affecting aggregation of the fluorophores and inducing the AIE effect (Figures 2b and c).
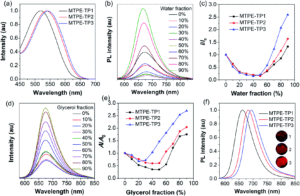
Figure 2: a) Absorption spectra of the fluorophore variants; b) photoluminescence spectra of the most conjugated variant, MTPE-TP3 with different water fractions; c) corresponding photoluminescence intensity plotted against water fractions for all three variants. d) to f) additionally indicate the effect of increased viscosity (and aggregation) upon glycerol addition to the fluorophores.
The researchers also formulated nanoparticles for each fluorophore variant to allow for better water solubility and therefore biocompatibility. They found that the absorption and emission of the nanoparticles became both brighter and more red-shifted and were now within the near-infrared range for favourable biological imaging. In vitro and in vivo testing of these nanoparticles in breast cancer cells and tumour-bearing mice verified that the AIE-nanoparticles are suitable for biological imaging, and indicate their potential to assist with tumour diagnosis in future clinical settings.
To find out more, please read:
Ji Qi, Xingchen Duan, Yuanjing Cai, Shaorui Jia, Chao Chen, Zheng Zhao, Ying Li, Hui-Qing Peng, Ryan T. K. Kwok, Jacky W. Y. Lam, Dan Ding and Ben Zhong Tang
Chem. Sci., 2020, 11, 8438-8447
About the blogger:
 Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.










