Low-valent compounds are attractive in chemical synthesis and catalysis due to their highly reactive nature. Carbenes are the archetypal example, where the carbon atom is divalent with two valence electrons, but group 15 analogues have also gained recent interest as reactive intermediates in fundamental transformations. These low-valent compounds (E-R), where the group 15 atom (E) has an oxidation state of +1 and is bound to just one additional atom, are extremely reactive and therefore challenging to isolate. The lighter congeners of nitrogen and phosphorus (as nitrenes, N-R, and phosphinidenes, P-R) have been isolated, but the heavier homologues are much more difficult to access and tend to undergo degradation. Stabilisation through adduct formation with Lewis bases had previously allowed for the formation of the heaviest group 15 bismuth homologue, and these stabilised bismuthinidenes showed potential in electrocatalytic and photophysical applications. Researchers in Germany and Switzerland have now reported for the first time the generation of a free and non-stabilised organometallic bismuthinidene compound, methylbismuth (BiMe), in the gas phase (Figure 1).
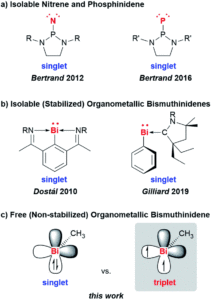
Figure 1: Structures and examples of low-valent group 15 compounds, with their electronic ground state configuration
The researchers targeted the non-stabilised organometallic bismuthinidene using a top-down approach, by breaking the Bi-C bonds of the higher valent and well-defined BiMe3 precursor (Scheme 1). They achieved this by pyrolysis of BiMe3, with subsequent analysis by photoelectron-photoion coincidence spectroscopy (PEPICO), that allows the recording of photoionisation mass spectra to detect ions produced by the pyrolysis. As shown by the photoionisation mass spectra in Figure 2, pyrolysis resulted in methyl loss through Bi-C homolytic cleavage, with higher pyrolysis power (bottom trace) showing full conversion of BiMe3 with by of m/z = 254. Stepwise methyl loss down to atomic bismuth was observed with m/z = 209 for Bi+, but notably BiMe+ was observed at m/z = 224, indicating bismuthinidene formation.
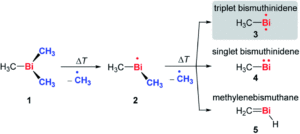
Scheme 1: Stepwise methyl abstraction from BiMe3 to generate bismuthinidene BiMe in the gas phase by flash pyrolysis
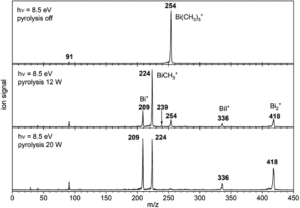
Figure 2: Photoionisation mass spectra showing methyl loss in the conversion of BiMe3 to BiMe by pyrolysis. Top trace = without pyrolysis, middle trace = low pyrolysis power, bottom trace = high pyrolysis power
The researchers further probed the electronic nature of the generated bismuthinidene by additional photoelectron spectroscopy and simulations. An ionisation energy of 7.88 eV was determined, and indicated the triplet (biradical) ground state (structure 3 in Scheme 1) as the lowest energy structure. This contrasts to the lighter N and P congeners, where the methylene species are the most energetically favoured (like 5 in Scheme 1). The researchers also conducted experiments to investigate the stepwise methyl abstraction via BiMe2•, determining a bond dissociation energy of 210 kJ mol-1 for the first Bi-C homolytic cleavage and demonstrating that this methyl abstraction could also be achieved under moderate reaction conditions. Overall, this report indicates that non-stabilised bismuthinidenes can be generated, with the potential for future exploitation as reactive intermediates in synthetic chemistry.
To find out more, please read:
Methylbismuth: an organometallic bismuthinidene biradical
Deb Pratim Mukhopadhyay, Domenik Schleier, Sara Wirsing, Jacqueline Ramler, Dustin Kaiser, Engelbert Reusch, Patrick Hemberger, Tobias Preitschopf, Ivo Krummenacher, Bernd Engels,* Ingo Fischer* and Crispin Lichtenberg*
Chem. Sci., 2020, Advance Article
About the blogger:
 Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.










