Intercalation of multivalent ions, e.g., Al3+, has received increasing attention as an energy-boosting strategy for rechargeable batteries. The charge storage process of the wide-spread Li-ion batteries relies on Li+ intercalating electrodes with layered structures. Multivalent-ion batteries could accommodate more charges than Li-ion batteries because their ions carry more charge than Li+. This concept, however, has now been challenged by a research team led by BenoÎt Limoges and Véronique Balland of Université de Paris, France. Their mechanistic investigation, published in Chemical Science, revealed that Al3+ ions were unable to intercalate electrodes in aqueous electrolytes.
The authors selected TiO2 arrays as the object of their study. These ~1 µm-high arrays were grown using the glancing angle deposition technique. By applying negative potentials to the TiO2 arrays, cations such as Al3+ could diffuse through the inter-array slits and interact with TiO2 (Figure 1).
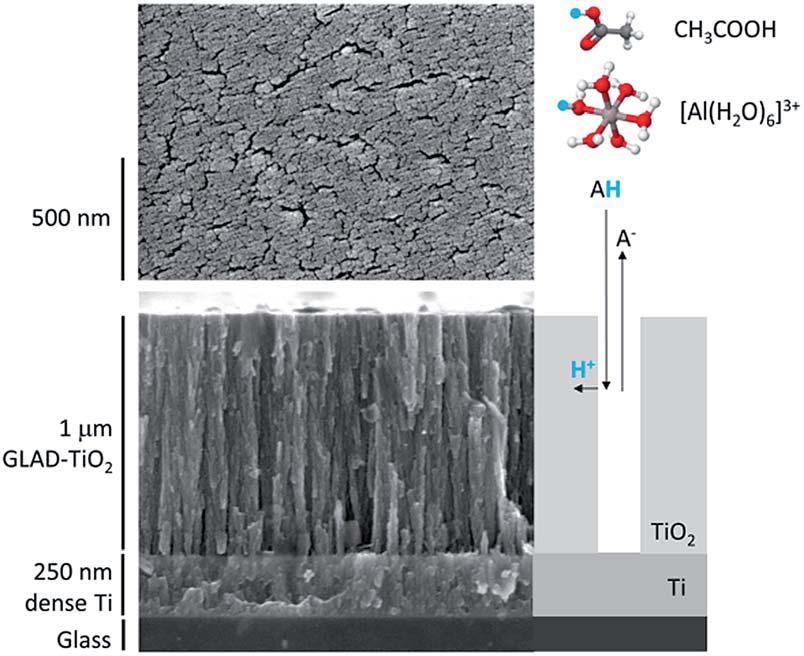
Figure 1. Structure of the TiO2 arrays. (left) Scanning electron micrographs and (right) a cartoon illustrating ion diffusion pathways.
Electrochemistry tests elucidated that the charge-storage process of TiO2 in an Al3+-containing aqueous electrolyte correlated to proton intercalation. This conclusion was mainly based on the nearly identical cyclic voltammograms (Figure 2, top) and capacity vs. potential curves (Figure 2, bottom) of the TiO2 arrays in both AlCl3 and acetic acid aqueous electrolytes. Since acetic acid solution had no Al3+, the observed charge-storage activity could not be attributed to Al3+ intercalation. Instead, the authors argued that protons dissociated either from hydrated Al3+ cations, [Al(H2O)6]3+ or acetic acid must intercalate TiO2 and result in the observed charge-storage capacities.
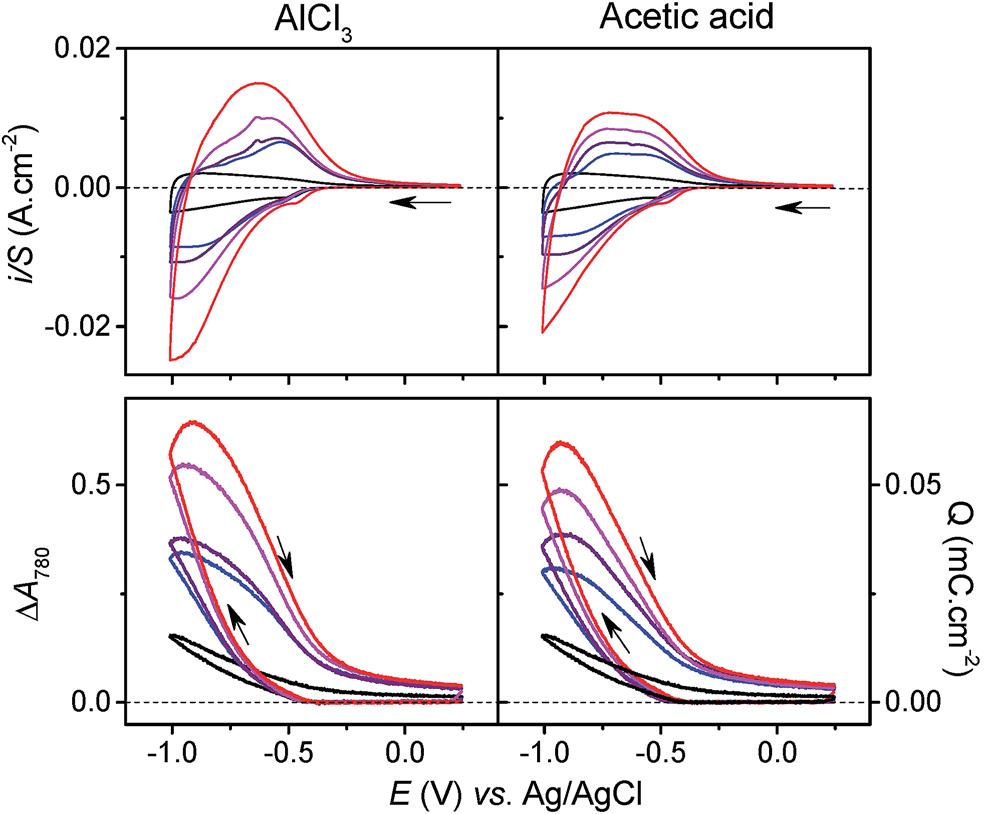
Figure 2. (top) Cyclic voltammograms and (bottom) capacity vs. potential curves of TiO2 in (left) Al3+-containing and (right) acetic acid aqueous electrolytes. The electrolytes are 0.3 M KCl with different concentrations of AlCl3 or acetic acid: 0 M (black), 25 mM (blue), 50 mM (purple), 100 mM (magenta), and 250 mM (red).
The authors believe that the misconception of Al3+ intercalation is due to the overlooking of Al3+ hydration, which is inevitable when Al3+ is present in aqueous electrolytes. Removing the water shell (a prerequisite for ion intercalation) is energy costly for Al3+ because of the strong binding force between water molecules and Al3+. Additionally, even if Al3+ ions intercalate TiO2, their movement is strongly hindered by Coulombic interactions within the TiO2 lattice. The immobilized intercalated ions would then block other ions from entering the TiO2 lattice. Together, both factors prevent Al3+ from intercalating into TiO2.
In summary, this work demonstrates that the charge-storage capacity of TiO2 in Al3+-containing aqueous electrolytes is most probably due to proton intercalation. This conclusion also applies to other multivalent cations, including Zn2+ and Mn2+, as shown in this work.
To find out more, please read:
Yee-Seul Kim, Kenneth D. Harris, BenoÎt Limoges, and Véronique Balland
Chem. Sci., 2019, doi: 10.1039/c9sc02397f
Tianyu Liu acknowledges Zachary L. Croft of Virginia Tech, the U.S., for his constructive comments on this post.
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about the communication of scientific endeavors and cutting-edge research to both the general public and other scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about the communication of scientific endeavors and cutting-edge research to both the general public and other scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.