We are happy to present a selection of our HOT articles for January. To see all of our HOT referee-recommended articles from 2019, please find the collection here.
As always, Chemical Science articles are free to access.
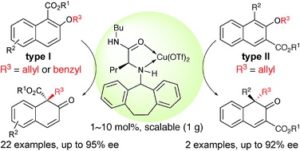
Lu Yao, Kazuaki Ishihara*
Chem. Sci., 2019, 10, 2259-2263
DOI: 10.1039/C8SC05601C, Edge Article
______________________________________________________
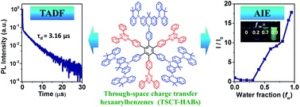
Xingdong Wang, Shumeng Wang, Jianhong Lv, Shiyang Shao,* Lixiang Wang,* Xiabin Jing and Fosong Wang
Chem. Sci., 2019, Advance Article
DOI: 10.1039/C8SC04991B, Edge Article
______________________________________________________
Luca Rocchigiani,* Peter H. M. Budzelaar* and Manfred Bochmann*
Chem. Sci., 2019, 10, 2633-2642
DOI: 10.1039/C8SC05229H, Edge Article
______________________________________________________
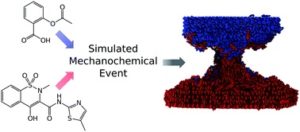
Michael Ferguson, M. Silvina Moyano, Gareth A. Tribello, Deborah E. Crawford, Eduardo M. Bringa, Stuart L. James,* Jorge Kohanoff* and Mario G. Del Pópolo*
Chem. Sci., 2019, Advance Article
DOI: 10.1039/C8SC04971H, Edge Article
______________________________________________________
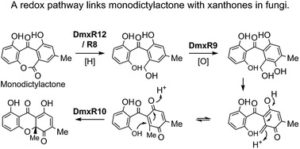
Claudio Greco, Kate de Mattos-Shipley, Andrew M. Bailey, Nicholas P. Mulholland, Jason L. Vincent, Christine L. Willis, Russell J. Cox* and Thomas J. Simpson*
Chem. Sci., 2019, Advance Article
DOI: 10.1039/C8SC05126G, Edge Article
______________________________________________________
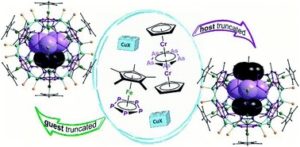
Helena Brake, Eugenia Peresypkina, Claudia Heindl, Alexander V. Virovets, Werner Kremer and Manfred Scheer*
Chem. Sci., 2019, Advance Article
DOI: 10.1039/C8SC05471A, Edge Article
______________________________________________________











 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at 
